Melphalan: Difference between revisions
No edit summary |
Gerald Chi (talk | contribs) mNo edit summary |
||
| Line 143: | Line 143: | ||
}} | }} | ||
[[Category: | [[Category:Chemotherapy]] | ||
[[Category:Chemotherapeutic agents]] | [[Category:Chemotherapeutic agents]] | ||
[[Category:Alkylating agents]] | |||
[[Category:Nitrogen mustards]] | |||
Latest revision as of 15:34, 23 February 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Warning: Severe bone marrow suppression
See full prescribing information for complete Boxed Warning.
Melphalan should be administered under the supervision of a qualified physician experienced in the use of cancer chemotherapeutic agents. Severe bone marrow suppression with resulting infection or bleeding may occur. Melphalan is leukemogenic in humans.
Melphalan produces chromosomal aberrations in vitro and in vivo and, therefore, should be considered potentially mutagenic in humans.
|
Overview
Melphalan is an alkylating agent that is FDA approved for the treatment of multiple myeloma and for the palliation of non-resectable epithelial carcinoma of the ovary. There is a Black Box Warning for this drug as shown here. Common adverse reactions include stomatitis.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
For the palliative treatment of multiple myeloma
- Dosage:
- The usual oral dose is 6 mg (3 tablets) daily. The entire daily dose may be given at one time.
- The dose is adjusted, as required, on the basis of blood counts done at approximately weekly intervals.
- After 2 to 3 weeks of treatment, the drug should be discontinued for up to 4 weeks, during which time the blood count should be followed carefully.
- When the white blood cell and platelet counts are rising, a maintenance dose of 2 mg daily may be instituted.
For the palliation of non-resectable epithelial carcinoma of the ovary.
- Dosage:
- 0.2 mg/kg daily for 5 days as a single course. Courses are repeated every 4 to 5 weeks depending upon hematologic tolerance.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Melphalan in adult patients.
Non–Guideline-Supported Use
- Dosage: 0.25 mg/kg/day for 4 to 7 days, repeated at 4 to 6 week interval
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness have not been established in pediatric patients
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Melphalan in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Melphalan in pediatric patients.
Contraindications
Melphalan should not be used in patients whose disease has demonstrated a prior resistance to this agent. Patients who have demonstrated hypersensitivity to melphalan should not be given the drug.
Warnings
|
Warning: Severe bone marrow suppression
See full prescribing information for complete Boxed Warning.
Melphalan should be administered under the supervision of a qualified physician experienced in the use of cancer chemotherapeutic agents. Severe bone marrow suppression with resulting infection or bleeding may occur. Melphalan is leukemogenic in humans.
Melphalan produces chromosomal aberrations in vitro and in vivo and, therefore, should be considered potentially mutagenic in humans.
|
As with other nitrogen mustard drugs, excessive dosage will produce marked bone marrow suppression.Bone marrow suppression is the most significant toxicity associated with melphalan in most patients. Therefore, the following tests should be performed at the start of therapy and prior to each subsequent course of melphalan: platelet count, hemoglobin, white blood cell count, and differential. Thrombocytopenia and/or leukopenia are indications to withhold further therapy until the blood counts have sufficiently recovered. Frequent blood counts are essential to determine optimal dosage and to avoid toxicity. Dose adjustment on the basis of blood counts at the nadir and day of treatment should be considered.
Hypersensitivity reactions, including anaphylaxis, have occurred rarely. These reactions have occurred after multiple courses of treatment and have recurred in patients who experienced a hypersensitivity reaction to IV melphalan. If a hypersensitivity reaction occurs, oral or IV melphalan should not be readministered.
Adverse Reactions
Clinical Trials Experience
Hematologic
The most common side effect is bone marrow suppression leading to leukopenia, thrombocytopenia, and anemia. Although bone marrow suppression frequently occurs, it is usually reversible if melphalan is withdrawn early enough. However, irreversible bone marrow failure has been reported.
Gastrointestinal
Nausea, vomiting, diarrhea, and oral ulceration occur. Hepatic disorders ranging from abnormal liver function tests to clinical manifestations such as hepatitis and jaundice have been reported.
Miscellaneous
Other reported adverse reactions include: pulmonary fibrosis (including fatal outcomes) and interstitial pneumonitis, skin hypersensitivity, maculopapular rashes, vasculitis, alopecia, and hemolytic anemia. Allergic reactions, including urticaria, edema, skin rashes, and rare anaphylaxis, have occurred after multiple courses of treatment. Cardiac arrest has also been reported rarely in association with such reports.
Postmarketing Experience
There is limited information regarding Melphalan Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Melphalan Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): D Melphalan may cause fetal harm when administered to a pregnant woman. Melphalan was embryolethal and teratogenic in rats following oral (6 to 18 mg/m2/day for 10 days) and intraperitoneal (18 mg/m2) administration. Malformations resulting from melphalan included alterations of the brain (underdevelopment, deformation, meningocele, and encephalocele) and eye (anophthalmia and microphthalmos), reduction of the mandible and tail, as well as hepatocele (exomphaly).
There are no adequate and well-controlled studies in pregnant women. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing potential should be advised to avoid becoming pregnant.
Pregnancy Category (AUS): D
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Melphalan in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Melphalan during labor and delivery.
Nursing Mothers
It is not known whether this drug is excreted in human milk. melphalan should not be given to nursing mothers.
Pediatric Use
The safety and effectiveness of melphalan in pediatric patients have not been established.
Geriatic Use
Clinical studies of melphalan Tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Melphalan with respect to specific gender populations.
Race
There is no FDA guidance on the use of Melphalan with respect to specific racial populations.
Renal Impairment
In patients with moderate to severe renal impairment, currently available pharmacokinetic data do not justify an absolute recommendation on dosage reduction to those patients, but it may be prudent to use a reduced dose initially.
Hepatic Impairment
There is no FDA guidance on the use of Melphalan in patients with hepatic impairment.
Females of Reproductive Potential and Males
Melphalan causes suppression of ovarian function in premenopausal women, resulting in amenorrhea in a significant number of patients. Reversible and irreversible testicular suppression have also been reported.
Immunocompromised Patients
There is no FDA guidance one the use of Melphalan in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
Monitoring
Bood counts done at approximately weekly intervals.
IV Compatibility
There is limited information regarding the compatibility of Melphalan and IV administrations.
Overdosage
Overdoses, including doses up to 50 mg/day for 16 days, have been reported. Immediate effects are likely to be vomiting, ulceration of the mouth, diarrhea, and hemorrhage of the gastrointestinal tract. The principal toxic effect is bone marrow suppression. Hematologic parameters should be closely followed for 3 to 6 weeks. An uncontrolled study suggests that administration of autologous bone marrow or hematopoietic growth factors (i.e., sargramostim, filgrastim) may shorten the period of pancytopenia. General supportive measures, together with appropriate blood transfusions and antibiotics, should be instituted as deemed necessary by the physician. This drug is not removed from plasma to any significant degree by hemodialysis.
Pharmacology
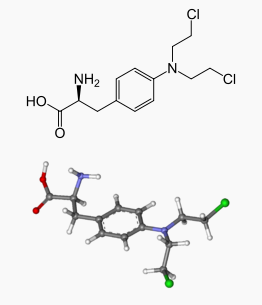
| |
Melphalan
| |
| Systematic (IUPAC) name | |
| 4-[bis(chloroethyl)amino]phenylalanine | |
| Identifiers | |
| CAS number | |
| ATC code | L01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 305.2 g/mol |
| SMILES | & |
| Synonyms | 2-amino-3-[4-[bis(2-chloroethyl)amino]phenyl]-propanoic acid |
| Pharmacokinetic data | |
| Bioavailability | 25% to 89% |
| Metabolism | hydrolysis |
| Half life | 1.5 ± 0.8 hours |
| Excretion | Renal, significantly metabolised |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
Template:Unicode Prescription only |
| Routes | Oral, intravenous |
Mechanism of Action
Melphalan is an alkylating agent of the bischloroethylamine type. As a result, its cytotoxicity appears to be related to the extent of its interstrand cross-linking with DNA, probably by binding at the N7 position of guanine. Like other bifunctional alkylating agents, it is active against both resting and rapidly dividing tumor cells.
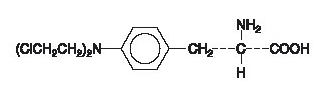
Structure
The molecular formula is C13H18Cl2N2O2 and the molecular weight is 305.20. The structural formula is:
Pharmacodynamics
There is limited information regarding Melphalan Pharmacodynamics in the drug label.
Pharmacokinetics
The absorption of oral melphalan is highly variable with respect to both the time to first appearance of the drug in plasma (range: 0 to 6 hours) and peak plasma concentration (Cmax). The average absolute bioavailability of melphalan is also highly variable (range: 56% to 93%). These results may be due to incomplete intestinal absorption, a variable “first pass” hepatic metabolism, or to rapid hydrolysis. Oral administration of melphalan with a high fat meal may reduce melphalan exposure (AUC) by 36% to 54%.
In 18 patients given a single oral dose of 0.6 mg/kg of melphalan, the terminal elimination plasma half-life (t1/2) of parent drug was 1.5 ± 0.83 hours. The 24-hour urinary excretion of parent drug in these patients was 10% ± 4.5%, suggesting that renal clearance is not a major route of elimination of parent drug. In a separate study in 18 patients given single oral doses of 0.2 to 0.25 mg/kg of melphalan, Cmax and plasma concentration-time curves (AUC), when dose adjusted to a dose of 14 mg, were (mean ± SD) 212 ± 74 ng/mL and 498 ± 137 ng•hr/mL, respectively. Elimination phase t½ in these patients was approximately 1 hour and the median tmax was 1 hour.
One study using universally labeled 14C-melphalan, found substantially less radioactivity in the urine of patients given the drug by mouth (30% of administered dose in 9 days) than in the urine of those given it intravenously (35% to 65% in 7 days). Following either oral or IV administration, the pattern of label recovery was similar, with the majority being recovered in the first 24 hours. Following oral administration, peak radioactivity occurred in plasma at 2 hours and then disappeared with a half-life of approximately 160 hours. In 1 patient where parent drug (rather than just radiolabel) was determined, the melphalan half-disappearance time was 67 minutes.
The steady-state volume of distribution of melphalan is 0.5 L/kg. Penetration into cerebrospinal fluid (CSF) is low. The average melphalan binding to plasma proteins is highly variable (range: 53% to 92%). Serum albumin is the major binding protein, accounting for approximately 40% to 60% of the plasma protein binding, while α1-acid glycoprotein accounts for about 20% of the plasma protein binding. Approximately 30% of melphalan is (covalently) irreversibly bound to plasma proteins. Interactions with immunoglobulins have been found to be negligible.
Melphalan is eliminated from plasma primarily by chemical hydrolysis to monohydroxymelphalan and dihydroxymelphalan. Aside from these hydrolysis products, no other melphalan metabolites have been observed in humans. Although the contribution of renal elimination to melphalan clearance appears to be low, one pharmacokinetic study showed a significant positive correlation between the elimination rate constant for melphalan and renal function and a significant negative correlation between renal function and the area under the plasma melphalan concentration/time curve.
Nonclinical Toxicology
Carcinogenesis
Secondary malignancies, including acute nonlymphocytic leukemia, myeloproliferative syndrome, and carcinoma have been reported in patients with cancer treated with alkylating agents (including melphalan). Some patients also received other chemotherapeutic agents or radiation therapy. Precise quantitation of the risk of acute leukemia, myeloproliferative syndrome, or carcinoma is not possible. Published reports of leukemia in patients who have received melphalan (and other alkylating agents) suggest that the risk of leukemogenesis increases with chronicity of treatment and with cumulative dose. In one study, the 10-year cumulative risk of developing acute leukemia or myeloproliferative syndrome after melphalan therapy was 19.5% for cumulative doses ranging from 730 mg to 9,652 mg. In this same study, as well as in an additional study, the 10-year cumulative risk of developing acute leukemia or myeloproliferative syndrome after melphalan therapy was less than 2% for cumulative doses under 600 mg. This does not mean that there is a cumulative dose below which there is no risk of the induction of secondary malignancy. The potential benefits from melphalan therapy must be weighed on an individual basis against the possible risk of the induction of a second malignancy.
Adequate and well-controlled carcinogenicity studies have not been conducted in animals. However, i.p. administration of melphalan in rats (5.4 to 10.8 mg/m2) and in mice (2.25 to 4.5 mg/m2) 3 times per week for 6 months followed by 12 months post-dose observation produced peritoneal sarcoma and lung tumors, respectively.
Mutagenesis
melphalan has been shown to cause chromatid or chromosome damage in humans. Intramuscular administration of melphalan at 6 and 60 mg/m2 produced structural aberrations of the chromatid and chromosomes in bone marrow cells of Wistar rats.
Clinical Studies
There is limited information regarding Melphalan Clinical Studies in the drug label.
How Supplied
Melphalan tablets 2 mg
- Bottle of 50 (NDC 52609-0001-5).
Storage
Store in a refrigerator, 2° to 8°C (36° to 46°F).
Images
Drug Images
{{#ask: Page Name::Melphalan |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Melphalan |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Patients should be informed that the major toxicities of melphalan are related to bone marrow suppression, hypersensitivity reactions, gastrointestinal toxicity, and pulmonary toxicity. The major long-term toxicities are related to infertility and secondary malignancies. Patients should never be allowed to take the drug without close medical supervision and should be advised to consult their physician if they experience skin rash, vasculitis, bleeding, fever, persistent cough, nausea, vomiting, amenorrhea, weight loss, or unusual lumps/masses. Women of childbearing potential should be advised to avoid becoming pregnant.
Precautions with Alcohol
Alcohol-Melphalan interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Alkeran [1]
Look-Alike Drug Names
There is limited information regarding Melphalan Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Melphalan |Label Name=Melphalan 2 mg.png
}}