Melanoma: Difference between revisions
| Line 38: | Line 38: | ||
[[Melanoma medical therapy|Medical therapy]] | [[Melanoma surgery|Surgical options]] | [[Melanoma metastasis treatment|Metastasis Treatment]] | [[Melanoma primary prevention|Primary prevention]] | [[Melanoma secondary prevention|Secondary prevention]] | [[Melanoma cost-effectiveness of therapy|Financial costs]] | [[Melanoma future or investigational therapies|Future therapies]] | [[Melanoma medical therapy|Medical therapy]] | [[Melanoma surgery|Surgical options]] | [[Melanoma metastasis treatment|Metastasis Treatment]] | [[Melanoma primary prevention|Primary prevention]] | [[Melanoma secondary prevention|Secondary prevention]] | [[Melanoma cost-effectiveness of therapy|Financial costs]] | [[Melanoma future or investigational therapies|Future therapies]] | ||
==Types of primary melanoma== | ==Types of primary melanoma== | ||
Revision as of 14:48, 21 December 2011
For patient information click here
| Melanoma | |
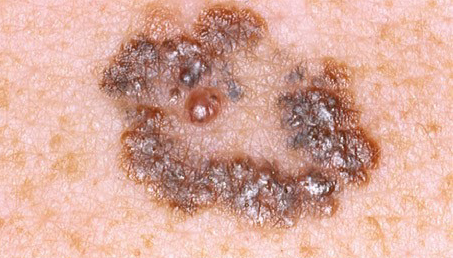 | |
|---|---|
| Melanoma malignum on the left leg of a 60-year-old woman | |
| ICD-10 | C43 |
| ICD-9 | 172 |
| ICD-O: | Template:ICDO |
| OMIM | 155600 |
| DiseasesDB | 7947 |
| MedlinePlus | 000850 |
| eMedicine | derm/257 |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [2] Please Join in Editing This Page and Apply to be an Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [3] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
|
Melanoma Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Melanoma On the Web |
|
American Roentgen Ray Society Images of Melanoma |
Overview
History
Epidemiology
The incidence of melanoma has increased in the recent years, but it is not clear to what extent changes in behavior, in the environment, or in early detection are involved.[1]
Causes
Risk factors
Pathophysiology
Diagnosis
- History and Symptoms | Physical Examination | Staging | Lab Tests | Electrocardiogram | X Ray | MRI | CT | Echocardiography | Other imaging findings | Other diagnostic studies
Treatment
Medical therapy | Surgical options | Metastasis Treatment | Primary prevention | Secondary prevention | Financial costs | Future therapies
Types of primary melanoma
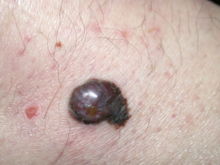
The most common types of Melanoma in the skin:
- superficial spreading melanoma (SSM)
- nodular melanoma
- acral lentiginous melanoma
- lentigo maligna (melanoma)
Any of the above types may produce melanin (and be dark in colour) or not (and be amelanotic - not dark). Similarly any subtype may show desmoplasia (dense fibrous reaction with neurotropism) which is a marker of aggressive behaviour and a tendency to local recurrence.
Elsewhere:
- clear cell sarcoma (Melanoma of Soft Parts)
- mucosal melanoma
- uveal melanoma
Prognostic factors
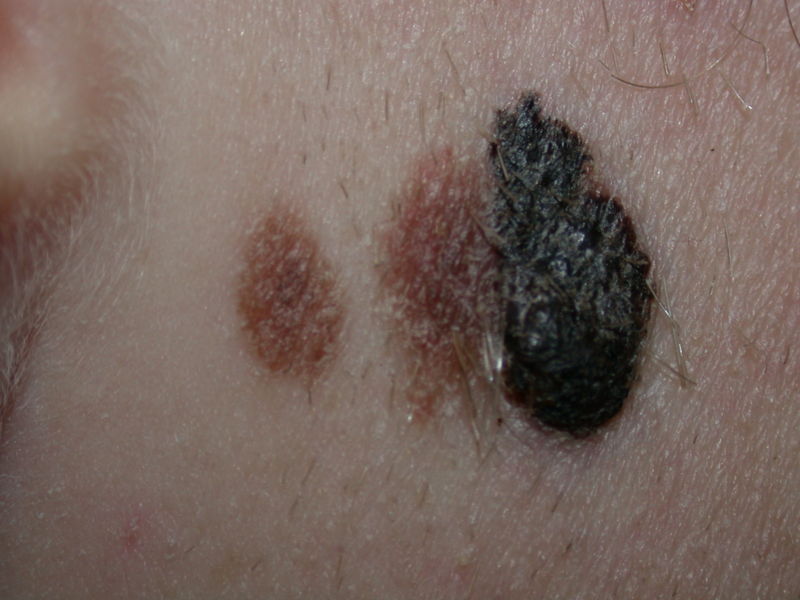
Features that affect prognosis are tumor thickness in millimeters (Breslow's depth), depth related to skin structures (Clark level), type of melanoma, presence of ulceration, presence of lymphatic/perineural invasion, presence of tumor infiltrating lymphocytes (if present, prognosis is better), location of lesion, presence of satellite lesions, and presence of regional or distant metastasis.[2]
Certain types of melanoma have worse prognoses but this is explained by their thickness. Interestingly, less invasive melanomas even with lymph node metastases carry a better prognosis than deep melanomas without regional metastasis at time of staging. Local recurrences tend to behave similarly to a primary unless they are at the site of a wide local excision (as opposed to a staged excision or punch/shave excision) since these recurrences tend to indicate lymphatic invasion.
When melanomas have spread to the lymph nodes, one of the most important factors is the number of nodes with malignancy. Extent of malignancy within a node is also important; micrometastases in which malignancy is only microscopic have a more favorable prognosis than macrometastases. In some cases micrometastases may only be detected by special staining, and if malignancy is only detectable by a rarely-employed test known as polymerase chain reaction (PCR), the prognosis is better. Macrometastases in which malignancy is clinically apparent (in some cases cancer completely replaces a node) have a far worse prognosis, and if nodes are matted or if there is extracapsular extension, the prognosis is still worse.
When there is distant metastasis, the cancer is generally considered incurable. The five year survival rate is less than 10%.[3] The median survival is 6 to 12 months. Treatment is palliative, focusing on life-extension and quality of life. In some cases, patients may live many months or even years with metastatic melanoma (depending on the aggressiveness of the treatment). Metastases to skin and lungs have a better prognosis. Metastases to brain, bone and liver are associated with a worse prognosis.
There is not enough definitive evidence to adequately stage, and thus give a prognosis for ocular melanoma and melanoma of soft parts, or mucosal melanoma (e.g. rectal melanoma), although these tend to metastasize more easily. Even though regression may increase survival, when a melanoma has regressed, it is impossible to know its original size and thus the original tumor is often worse than a pathology report might indicate.
Staging
Further context on cancer staging is available at TNM.
Also of importance are the "Clark level" and "Breslow depth" which refer to the microscopic depth of tumor invasion.[4]
Melanoma stages:[3]
Stage 0: Melanoma in Situ (Clark Level I), 100% Survival
Stage I/II: Invasive Melanoma, 85-95% Survival
- T1a: Less than 1.00 mm primary, w/o Ulceration, Clark Level II-III
- T1b: Less than 1.00 mm primary, w/Ulceration or Clark Level IV-V
- T2a: 1.00-2.00 mm primary, w/o Ulceration
Stage II: High Risk Melanoma, 40-85% Survival
- T2b: 1.00-2.00 mm primary, w/ Ulceration
- T3a: 2.00-4.00 mm primary, w/o Ulceration
- T3b: 2.00-4.00 mm primary, w/ Ulceration
- T4a: 4.00 mm or greater primary w/o Ulceration
- T4b: 4.00 mm or greater primary w/ Ulceration
Stage III: Regional Metastasis, 25-60% Survival
- N1: Single Positive Lymph Node
- N2: 2-3 Positive Lymph Nodes OR Regional Skin/In-Transit Metastasis
- N3: 4 Positive Lymph Nodes OR Lymph Node and Regional Skin/In Transit Metastases
Stage IV: Distant Metastasis, 9-15% Survival
- M1a: Distant Skin Metastasis, Normal LDH
- M1b: Lung Metastasis, Normal LDH
- M1c: Other Distant Metastasis OR Any Distant Metastasis with Elevated LDH
Based Upon AJCC 5-Year Survival With Proper Treatment
Treatment
Treatment of advanced malignant melanoma is performed from a multidisciplinary approach including dermatologists, medical oncologists, radiation oncologists, surgical oncologists, general surgeons, plastic surgeons, neurologists, neurosurgeons, otorhinolaryngologists, radiologists, pathologists/dermatopathologists, research scientists, nurse practitioners and physician assistants, and palliative care experts. Nurse practitioners (NPs) and physician assistants (PAs) are qualified to evaluate and treat patients on behalf of their supervising physicians. Treatment guidelines can be found through many resources available to health care professionals around the world. Inspired by melanoma’s increasing prevalence, researchers are seeking to understand the pathways that regulate melanin production.
Surgery

Diagnostic punch or excisional biopsies may appear to excise (and in some cases may indeed actually remove) the tumor, but further surgery is often necessary to reduce the risk of recurrence.
Complete surgical excision with adequate margins and assessment for the presence of detectable metastatic disease along with short and long term follow up is standard. Often this is done by a "wide local excision" (WLE) with 1 to 2 cm margins. The wide excision aims to reduce the rate of tumour recurrence at the site of the original lesion. This is a common pattern of treatment failure in melanoma. Considerable research has aimed to elucidate appropriate margins for excision with a general trend toward less aggressive treatment during the last decades. There seems to be no advantage to taking in excess of 2 cm margins for even the thickest tumors.[5]
Mohs micrographic surgery is not well accepted in the treatment of melanoma. In this surgery, performed by specially-trained dermatologists, a small layer of tissue is excised and prepared as a frozen tissue section. This section can be prepared and examined by the dermatologist/dermatopathologist within one hour, and the patient will return for further stages of excision as needed, with each excised tissue layer being examined until clear margins are obtained.[6] However, the usefulness of Moh's surgery in melanoma is limited because of the difficulty of identifying melanocytic atypia on a frozen section, which may lead to incomplete resection of the melanoma.[6][7]
Other issues to consider with Moh's technique are risks of tumor implantation and possible false negative margins due to suboptimal melanocytic staining.[8] Deviation from recommended 1-2 cm margins of excision should thus be approached carefully.
Melanomas which spread usually do so to the lymph nodes in the region of the tumour before spreading elsewhere. Attempts to improve survival by removing lymph nodes surgically (lymphadenectomy) were associated with many complications but unfortunately no overall survival benefit. Recently the technique of sentinel lymph node biopsy has been developed to reduce the complications of lymph node surgery while allowing assessment of the involvement of nodes with tumour.
Although controversial and without prolonging survival, "sentinel lymph node" biopsy is often performed, especially for T1b/T2+ tumors, mucosal tumors, ocular melanoma and tumors of the limbs. A process called lymphoscintigraphy is performed in which a radioactive tracer is injected at the tumor site in order to localize the "sentinel node(s)". Further precision is provided using a blue tracer dye and surgery is performed to biopsy the node(s). Routine H&E staining, and immunoperoxidase staining will be adequate to rule out node involvement. PCR (Polymerase Chain Reaction) tests on nodes, usually performed to test for entry into clinical trials, now demonstrate that many patients with a negative SLN actually had a small number of positive cells in their nodes. Alternatively, a fine-needle aspiration may be performed, and is often used to test masses.
If a lymph node is positive, depending on the extent of lymph node spread, a radical lymph node dissection will often be performed. If the disease is completely resected the patient will be considered for adjuvant therapy.
Adjuvant treatment
High risk melanomas may require referral to a medical or surgical oncologist for adjuvant treatment. In the United States most patients in otherwise good health will begin up to a year of high-dose interferon treatment, which has severe side effects, but may improve the patients' prognosis.[9] This claim is not supported by all research at this time and in Europe interferon is usually not used outside the scope of clinical trials.[10][11]
Metastatic melanomas can be detected by X-rays, CT scans, MRIs, PET and PET/CTs, ultrasound, LDH testing and photoacoustic detection.[12]
Chemotherapy and immunotherapy
Various chemotherapy agents are used, including dacarbazine (also termed DTIC), immunotherapy (with interleukin-2 (IL-2) or interferon (IFN)) as well as local perfusion are used by different centers. They can occasionally show dramatic success, but the overall success in metastatic melanoma is quite limited.[13] IL-2 (Proleukin®) is the first new therapy approved for the treatment of metastatic melanoma in 20 years. Studies have demonstrated that IL-2 offers the possibility of a complete and long-lasting remission in this disease, although only in a small percentage of patients.[14] A number of new agents and novel approaches are under evaluation and show promise.[15]
Lentigo maligna treatment
Some superficial melanomas (lentigo maligna) have resolved with an experimental treatment, imiquimod (Aldara®) topical cream, an immune enhancing agent. Application of this cream has been shown to decrease tumor size prior to surgery, reducing the invasiveness of the procedure. This treatment is used especially for smaller melanoma in situ lesions located in cosmetically sensitive regions. Several published studies demonstrate a 70% cure rate with this topical treatment. With lentigo maligna, surgical cure rates are no higher. Some dermasurgeons are combining the 2 methods: surgically excise the cancer, then treat the area with Aldara® cream post-operatively for 3 months.
Radiation and other therapies
Radiation therapy is often used after surgical resection for patients with locally or regionally advanced melanoma or for patients with unresectable distant metastases. It may reduce the rate of local recurrence but does not prolong survival.[16]
In research setting other therapies, such as gene therapy, may be tested.[17] Radioimmunotherapy of metastatic melanoma is currently under investigation.
Experimental treatment developed at the National Cancer Institute (NCI), part of the National Institutes of Health in the US was used in advanced (metastatic) melanoma with moderate success. The treatment, adoptive transfer of genetically altered autologous lymphocytes, depends on delivering genes that encode so called T cell receptors (TCRs), into patient's lymphocytes. After that manipulation lymphocytes recognize and bind to certain molecules found on the surface of melanoma cells and kill them.[18]
Equine melanoma
Melanomas are also not uncommon in horses, being largely confined to grey (or white) animals - 80% of such pale horses will develop melanomata by 15 years of age[19]; of these, 66% are slow growing but all may be classified as malignant[19]. Surgical excision may be attempted in some cases, if the tumours are limited in extent and number. However, they are often multiple (especially in older animals) and perineal tumours are notoriously difficult to excise. Often, a position of "benign neglect" is assumed, especially if the tumours are not causing any clinical problems. Medical therapy with cimetidine (2.5-4.0mg/kg three times daily for 2 months or more)[20] is also an option, although it has a lower success rate than surgery and cryosurgery[21].
Future thought
One important pathway in melanin synthesis involves the transcription factor MITF. The MITF gene is highly conserved and is found in people, mice, birds, and even fish. MITF production is regulated via a fairly straightforward pathway. UV radiation causes increased expression of transcription factor p53 in keratinocytes, and p53 causes these cells to produce melanoctye stimulating hormone (MSH), which binds to MC1R receptors on melanocytes. Ligand-binding at MC1R receptors activates adenyl cyclases, which produce cAMP, which activates CREB, which promotes MITF expression. The targets of MITF include p16 (a CDK inhibitor) and Bcl2, a gene essential to melanocyte survival. It is often difficult to design drugs that interfere with transcription factors, but perhaps new drugs will be discovered that can impede some reaction in the pathway upstream of MITF.
Studies of chromatin structure also promise to shed light on transcriptional regulation in melanoma cells. It has long been assumed that nucleosomes are positioned randomly on DNA, but murine studies of genes involved in melanin production now suggest that nucleosomes are stereotypically positioned on DNA. When a gene is undergoing transcription, its transcription start site is almost always nucleosome-free. When the gene is silent, however, nucleosomes often block the transcriptional start site, suggesting the nucleosome position may play a role in gene regulation.
Finally, given the fact that tanning helps protect skin cells from UV-induced damage, new melanoma prevention strategies could involve attempts to induce tanning in individuals who would otherwise get sunburns. Redheads, for example, do not tan because they have MC1R mutations. In mice, it has been shown that the melanin-production pathway can be rescued downstream of MC1R. Perhaps such a strategy will eventually be used to protect humans from melanoma.
References
- ↑ Berwick M, Wiggins C. "The current epidemiology of cutaneous malignant melanoma". Front Biosci. 11: 1244–54. PMID 16368510.
- ↑ Homsi J, Kashani-Sabet M, Messina J, Daud A (2005). "Cutaneous melanoma: prognostic factors". Cancer Control. 12 (4): 223–9. PMID 16258493.Full text (PDF)
- ↑ 3.0 3.1 Balch C, Buzaid A, Soong S, Atkins M, Cascinelli N, Coit D, Fleming I, Gershenwald J, Houghton A, Kirkwood J, McMasters K, Mihm M, Morton D, Reintgen D, Ross M, Sober A, Thompson J, Thompson J (2001). "Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma". J Clin Oncol. 19 (16): 3635–48. PMID 11504745.Full text
- ↑ Malignant melanoma: staging at Collaborative Hypertext of Radiology
- ↑ Balch C, Urist M, Karakousis C, Smith T, Temple W, Drzewiecki K, Jewell W, Bartolucci A, Mihm M, Barnhill R (1993). "Efficacy of 2-cm surgical margins for intermediate-thickness melanomas (1 to 4 mm). Results of a multi-institutional randomized surgical trial". Ann Surg. 218 (3): 262–7, discussion 267-9. PMID 8373269.
- ↑ 6.0 6.1 Bowen G, White G, Gerwels J (2005). "Mohs micrographic surgery". Am Fam Physician. 72 (5): 845–8. PMID 16156344.Full text
- ↑ Nahabedian M (2005). "Melanoma". Clin Plast Surg. 32 (2): 249–59. PMID 15814121.
- ↑ Nahabedian MY (2005). "Melanoma". Clin Plastic Surg. 32: 249–259.
- ↑ Kirkwood J, Strawderman M, Ernstoff M, Smith T, Borden E, Blum R (1996). "Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684". J Clin Oncol. 14 (1): 7–17. PMID 8558223.
- ↑ Kirkwood J, Ibrahim J, Sondak V, Richards J, Flaherty L, Ernstoff M, Smith T, Rao U, Steele M, Blum R (2000). "High- and low-dose interferon alfa-2b in high-risk melanoma: first analysis of intergroup trial E1690/S9111/C9190". J Clin Oncol. 18 (12): 2444–58. PMID 10856105.
- ↑ Kirkwood J, Ibrahim J, Sondak V, Ernstoff M, Ross M (2002). "Interferon alfa-2a for melanoma metastases". Lancet. 359 (9310): 978–9. PMID 11918944.
- ↑ Weight RM, Viator JA, Dale PS, Caldwell CW, Lisle AE. (2006). "Photoacoustic detection of metastatic melanoma cells in the human circulatory system". Opt Lett. 31 (20): 2998–3000. PMID 17001379.
- ↑ Bajetta E, Del Vecchio M, Bernard-Marty C, Vitali M, Buzzoni R, Rixe O, Nova P, Aglione S, Taillibert S, Khayat D (2002). "Metastatic melanoma: chemotherapy". Semin Oncol. 29 (5): 427–45. PMID 12407508.
- ↑ Buzaid A (2004). "Management of metastatic cutaneous melanoma". Oncology (Williston Park). 18 (11): 1443–50, discussion 1457-9. PMID 15609471.
- ↑ Danson S, Lorigan P (2005). "Improving outcomes in advanced malignant melanoma: update on systemic therapy". Drugs. 65 (6): 733–43. PMID 15819587.
- ↑ Bastiaannet E, Beukema J, Hoekstra H (2005). "Radiation therapy following lymph node dissection in melanoma patients: treatment, outcome and complications". Cancer Treat Rev. 31 (1): 18–26. PMID 15707701.
- ↑ Sotomayor M, Yu H, Antonia S, Sotomayor E, Pardoll D. "Advances in gene therapy for malignant melanoma". Cancer Control. 9 (1): 39–48. PMID 11907465.Full text (PDF)
- ↑ Press release from the NIH
- ↑ 19.0 19.1 Centre for Comparitive Oncology [1], accessed at 2220 on 12th July
- ↑ Warnick, LD, Graham, ME, and Valentine, BA (1995) "Evaluation of cimetidine treatment for melanomas in seven horses" Equine Practice, 17(7): 16-22, 1995
- ↑ RJ Rose & DR Hodson, Manual of Equine Practice (p. 498) 2000
External links
Websites
- Melanoma Molecular Map Project
- Proleukin
- Melanoma Perspectives
- Information on Melanoma from The Skin Cancer Foundation
- CIMIT Center for Integration of Medicine and Innovative Technology - New Advances and Research in Melanoma
- Sunbathing helps prevent cancer: UK newspaper article
- Melanoma International Foundation
- Melanoma Education Foundation
- melanoma.com (commercially supported site)
- DermNet NZ: Melanoma
- Professional melanoma information
- Adelaide Melanoma Unit (free information on diagnosis, prevention, treatment of melanoma; booklet available at cost)
- Assessing health risks of sunbeds and UV exposure summary by GreenFacts of the European Commission SCCP assessment
Patient information
- What You Need To Know About Moles and Dysplastic Nevi - patient information booklet from cancer.gov (PDF)
- MPIP: Melanoma patients information page
- Melanoma Support Organisation (Victoria, Australia) - Ran by Melanoma Sufferers with strong links to Cancer Institutes in Victoria, Australia
- Melanoma Patients Australia
- Mikes Page - The Melanoma Resource Center
- MEL-L - Melanoma e-mail list for patients, caregivers and healthcare professionals - Supporting the Melanoma Patient since 1996
Images, photographs
- Melanoma photo library at Dermnet
- DermAtlas: Melanoma images
- Photographs of melanoma
- Skin imaging methods for melanoma diagnosis(commercial advertising)
- Pictures of melanomas
- Pictures of amelanotic melanomas
Videos
- Health Video: Melanoma and Non-Melanoma Skin Cancers - Overview, Prevention, and Treatment
- Health Video: How to Perform a Skin Self Exam
be-x-old:Мэлянома bg:Меланома ca:Melanoma da:Malignt melanom de:Malignes Melanom et:Melanoom gl:Melanoma maligno it:Melanoma he:מלנומה la:Melanoma Malignus nl:Melanoom no:Malignt melanom sr:Меланом fi:Melanooma sv:Malignt melanom