Medroxyprogesterone acetate (injection): Difference between revisions
Shanshan Cen (talk | contribs) No edit summary |
m (Protected "Medroxyprogesterone acetate (injection)": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
(No difference)
| |
Latest revision as of 16:39, 20 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Shanshan Cen, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: LOSS OF BONE MINERAL DENSITY:
See full prescribing information for complete Boxed Warning.
Women who use medroxyprogesterone acetate injectable suspension may lose significant bone mineral density. Bone loss is greater with increasing duration of use and may not be completely reversible.
It is unknown if use of medroxyprogesterone acetate injectable suspension during adolescence or early adulthood, a critical period of bone accretion, will reduce peak bone mass and increase the risk for osteoporotic fracture in later life. Medroxyprogesterone acetate injectable suspension should not be used as a long-term birth control method (i.e., longer than 2 years) unless other birth control methods are considered inadequate. |
Overview
Medroxyprogesterone acetate (injection) is a progestin that is FDA approved for the prevention of pregnancy. There is a Black Box Warning for this drug as shown here. Common adverse reactions include injection site reaction, weight gain , abdominal pain, dizziness, headache, feeling nervous , amenorrhea, disorder of menstruation, menstrual spotting, reduced libido, fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
Medroxyprogesterone Acetate Injectable Suspension USP is indicated only for the prevention of pregnancy.
Dosage
Prevention of Pregnancy
The 1 mL vial of medroxyprogesterone acetate injectable suspension should be vigorously shaken just before use to ensure that the dose being administered represents a uniform suspension. The recommended dose is 150 mg of medroxyprogesterone acetate injectable suspension every 3 months (13 weeks) administered by deep IM injection in the gluteal or deltoid muscle. Medroxyprogesterone acetate injectable suspension should not be used as a long-term birth control method (i.e., longer than 2 years) unless other birth control methods are considered inadequate. Dosage does not need to be adjusted for body weight.
To ensure the patient is not pregnant at the time of the first injection, the first injection should be given ONLY during the first 5 days of a normal menstrual period; ONLY within the first 5-days postpartum if not breast-feeding; and if exclusively breast-feeding, ONLY at the sixth postpartum week. If the time interval between injections is greater than 13 weeks, the physician should determine that the patient is not pregnant before administering the drug. The efficacy of medroxyprogesterone acetate injectable suspension depends on adherence to the dosage schedule of administration.
Switching from other Methods of Contraception
When switching from other contraceptive methods, medroxyprogesterone acetate injectable suspension should be given in a manner that ensures continuous contraceptive coverage based upon the mechanism of action of both methods, (e.g., patients switching from oral contraceptives should have their first injection of medroxyprogesterone acetate injectable suspension on the day after the last active tablet or at the latest, on the day following the final inactive tablet).
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Medroxyprogesterone acetate (injection) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Medroxyprogesterone acetate (injection) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Medroxyprogesterone acetate (injection) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Medroxyprogesterone acetate (injection) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Medroxyprogesterone acetate (injection) in pediatric patients.
Contraindications
The use of medroxyprogesterone acetate injectable suspension is contraindicated in the following conditions:
- Known or suspected pregnancy or as a diagnostic test for pregnancy.
- Active thrombophlebitis, or current or past history of thromboembolic disorders, or cerebral vascular disease.
- Known or suspected malignancy of breast.
- Known hypersensitivity to medroxyprogesterone acetate injectable suspension or any of its other ingredients.
- Significant liver disease.
- Undiagnosed vaginal bleeding.
Warnings
|
WARNING: LOSS OF BONE MINERAL DENSITY:
See full prescribing information for complete Boxed Warning.
Women who use medroxyprogesterone acetate injectable suspension may lose significant bone mineral density. Bone loss is greater with increasing duration of use and may not be completely reversible.
It is unknown if use of medroxyprogesterone acetate injectable suspension during adolescence or early adulthood, a critical period of bone accretion, will reduce peak bone mass and increase the risk for osteoporotic fracture in later life. Medroxyprogesterone acetate injectable suspension should not be used as a long-term birth control method (i.e., longer than 2 years) unless other birth control methods are considered inadequate. |
1. Loss of Bone Mineral Density
Use of medroxyprogesterone acetate injectable suspension reduces serum estrogen levels and is associated with significant loss of bone mineral density (BMD). This loss of BMD is of particular concern during adolescence and early adulthood, a critical period of bone accretion. It is unknown if use of medroxyprogesterone acetate injectable suspension by younger women will reduce peak bone mass and increase the risk for osteoporotic fracture in later life.
After discontinuing medroxyprogesterone acetate injectable suspension in adolescents, mean BMD loss at total hip and femoral neck did not fully recover by 60 months (240 weeks) post-treatment. Similarly, in adults, there was only partial recovery of mean BMD at total hip, femoral neck and lumbar spine towards baseline by 24 months post-treatment.
Medroxyprogesterone acetate injectable suspension should not be used as a long-term birth control method (i.e., longer than 2 years) unless other birth control methods are considered inadequate. BMD should be evaluated when a woman needs to continue to use medroxyprogesterone acetate injectable suspension long-term. In adolescents, interpretation of BMD results should take into account patient age and skeletal maturity.
Other birth control methods should be considered in the risk/benefit analysis for the use of medroxyprogesterone acetate injectable suspension in women with osteoporosis risk factors. Medroxyprogesterone acetate injectable suspension can pose an additional risk in patients with risk factors for osteoporosis (e.g., metabolic bone disease, chronic alcohol and/or tobacco use, anorexia nervosa, strong family history of osteoporosis or chronic use of drugs that can reduce bone mass such as anticonvulsants or corticosteroids). Although there are no studies addressing whether calcium and Vitamin D may lessen BMD loss in women using medroxyprogesterone acetate injectable suspension, all patients should have adequate calcium and Vitamin D intake.
2. Thromboembolic Disorders
There have been reports of serious thrombotic events in women using medroxyprogesterone acetate injectable suspension (150 mg). However, medroxyprogesterone acetate injectable suspension has not been causally associated with the induction of thrombotic or thromboembolic disorders. Any patient who develops thrombosis while undergoing therapy with medroxyprogesterone acetate injectable suspension should discontinue treatment unless she has no other acceptable options for birth control.
Do not re-administer medroxyprogesterone acetate injectable suspension pending examination if there is a sudden partial or complete loss of vision or if there is a sudden onset of proptosis, diplopia, or migraine. Do not re-administer if examination reveals papilledema or retinal vascular lesions.
3. Cancer Risks
Breast Cancer
Women who have or have had a history of breast cancer should not use hormonal contraceptives, including medroxyprogesterone acetate injectable suspension, because breast cancer may be hormonally sensitive. Women with a strong family history of breast cancer should be monitored with particular care.
The results of five large case-control studies1, 2, 3, 4, 5 assessing the association between depomedroxyprogesterone acetate (DMPA) use and the risk of breast cancer are summarized in Figure 1. Three of the studies suggest a slightly increased risk of breast cancer in the overall population of users; these increased risks were statistically significant in one study. One recent US study1 evaluated the recency and duration of use and found a statistically significantly increased risk of breast cancer in recent users (defined as last use within the past five years) who used DMPA for 12 months or longer; this is consistent with results of a previous study4.
Figure 1 Risk estimates for breast cancer in DMPA users
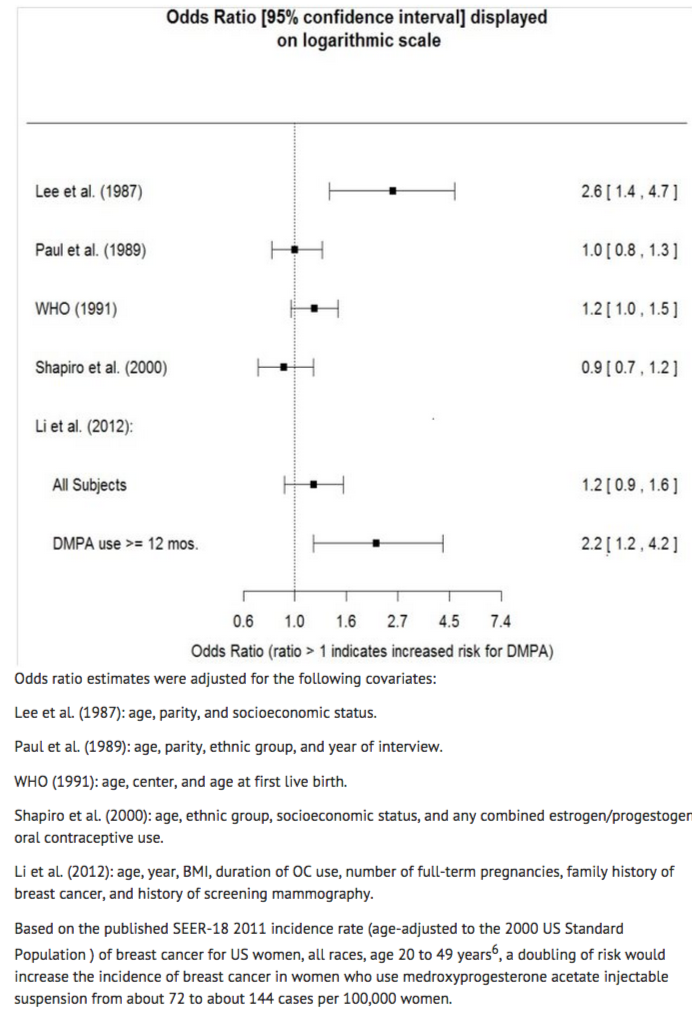
Cervical Cancer
A statistically nonsignificant increase in RR estimates of invasive squamous-cell cervical cancer has been associated with the use of medroxyprogesterone acetate injectable suspension in women who were first exposed before the age of 35 years (RR 1.22 to 1.28 and 95% CI 0.93 to 1.70). The overall, nonsignificant relative rate of invasive squamous-cell cervical cancer in women who ever used medroxyprogesterone acetate injectable suspension was estimated to be 1.11 (95% CI 0.96 to 1.29). No trends in risk with duration of use or times since initial or most recent exposure were observed.
Other Cancers
Long-term case-controlled surveillance of users of medroxyprogesterone acetate injectable suspension found no overall increased risk of ovarian or liver cancer.
4. Ectopic Pregnancy
Be alert to the possibility of an ectopic pregnancy among women using medroxyprogesterone acetate injectable suspension who become pregnant or complain of severe abdominal pain.
5. Anaphylaxis and Anaphylactoid Reaction
Anaphylaxis and anaphylactoid reaction have been reported with the use of medroxyprogesterone acetate injectable suspension. Institute emergency medical treatment if an anaphylactic reaction occurs.
6. Liver Function
Discontinue medroxyprogesterone acetate injectable suspension use if jaundice or acute or chronic disturbances of liver function develop. Do not resume use until markers of liver function return to normal and medroxyprogesterone acetate injectable suspension causation has been excluded.
7. Convulsions
There have been a few reported cases of convulsions in patients who were treated with medroxyprogesterone acetate injectable suspension. Association with drug use or pre-existing conditions is not clear.
8. Depression
Monitor patients who have a history of depression and do not readminister medroxyprogesterone acetate injectable suspension if depression recurs.
9. Bleeding Irregularities
Most women using medroxyprogesterone acetate injectable suspension experience disruption of menstrual bleeding patterns. Altered menstrual bleeding patterns include amenorrhea, irregular or unpredictable bleeding or spotting, prolonged spotting or bleeding, and heavy bleeding. Rule out the possibility of organic pathology if abnormal bleeding persists or is severe, and institute appropriate treatment.
As women continue using medroxyprogesterone acetate injectable suspension, fewer experience irregular bleeding and more experience amenorrhea. In clinical studies of medroxyprogesterone acetate injectable suspension, by month 12 amenorrhea was reported by 55% of women, and by month 24, amenorrhea was reported by 68% of women using medroxyprogesterone acetate injectable suspension.
10. Weight Gain
Women tend to gain weight while on therapy with medroxyprogesterone acetate injectable suspension. From an initial average body weight of 136 lb, women who completed 1 year of therapy with medroxyprogesterone acetate injectable suspension gained an average of 5.4 lb. Women who completed 2 years of therapy gained an average of 8.1 lb. Women who completed 4 years gained an average of 13.8 lb. Women who completed 6 years gained an average of 16.5 lb. Two percent of women withdrew from a large-scale clinical trial because of excessive weight gain.
11. Carbohydrate Metabolism
A decrease in glucose tolerance has been observed in some patients on medroxyprogesterone acetate injectable suspension treatment. Monitor diabetic patients carefully while receiving medroxyprogesterone acetate injectable suspension.
12. Lactation
Detectable amounts of drug have been identified in the milk of mothers receiving medroxyprogesterone acetate injectable suspension. In nursing mothers treated with medroxyprogesterone acetate injectable suspension, milk composition, quality, and amount are not adversely affected. Neonates and infants exposed to medroxyprogesterone from breast milk have been studied for developmental and behavioral effects through puberty. No adverse effects have been noted.
13. Fluid Retention
Because progestational drugs including medroxyprogesterone acetate injectable suspension may cause some degree of fluid retention, monitor patients with conditions that might be influenced by this condition, such as epilepsy, migraine, asthma, and cardiac or renal dysfunction.
14. Return of Fertility
Return to ovulation and fertility is likely to be delayed after stopping medroxyprogesterone acetate injectable suspension. In a large US study of women who discontinued use of medroxyprogesterone acetate injectable suspension to become pregnant, data are available for 61% of them. Of the 188 women who discontinued the study to become pregnant, 114 became pregnant. Based on Life-Table analysis of these data, it is expected that 68% of women who do become pregnant may conceive within 12 months, 83% may conceive within 15 months, and 93% may conceive within 18 months from the last injection. The median time to conception for those who do conceive is 10 months following the last injection with a range of 4 to 31 months, and is unrelated to the duration of use. No data are available for 39% of the patients who discontinued medroxyprogesterone acetate injectable suspension to become pregnant and who were lost to follow-up or changed their mind.
15. Sexually Transmitted Diseases
Patients should be counseled that medroxyprogesterone acetate injectable suspension does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
16. Pregnancy
Although medroxyprogesterone acetate injectable suspension should not be used during pregnancy, there appears to be little or no increased risk of birth defects in women who have inadvertently been exposed to medroxyprogesterone acetate injections in early pregnancy. Neonates exposed to medroxyprogesterone acetate in-utero and followed to adolescence showed no evidence of any adverse effects on their health including their physical, intellectual, sexual or social development.
Adverse Reactions
Clinical Trials Experience
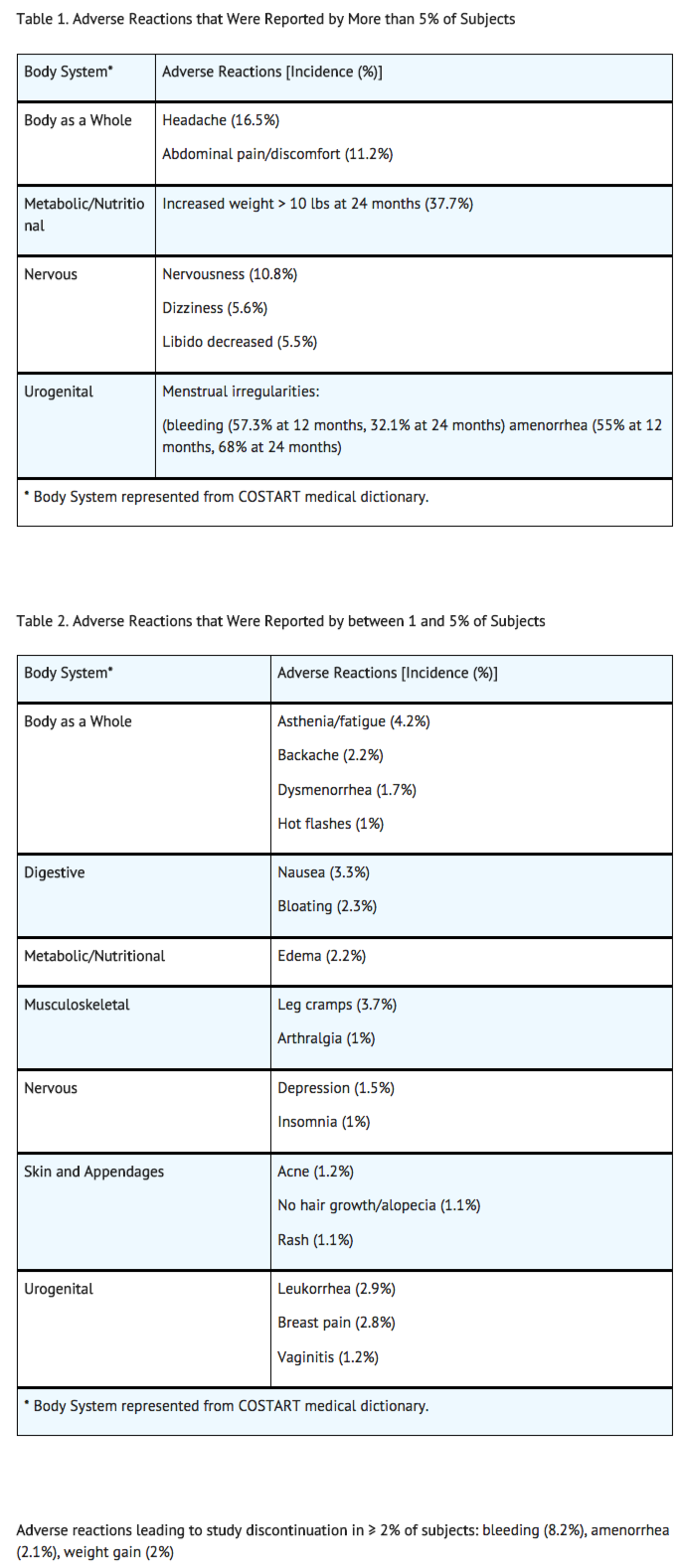
Postmarketing Experience

Drug Interactions
Changes in Contraceptive Effectiveness Associated with Coadministration of Other Products
If a woman on hormonal contraceptives takes a drug or herbal product that induces enzymes, including CYP3A4, that metabolize contraceptive hormones, counsel her to use additional contraception or a different method of contraception. Drugs or herbal products that induce such enzymes may decrease the plasma concentrations of contraceptive hormones, and may decrease the effectiveness of hormonal contraceptives. Some drugs or herbal products that may decrease the effectiveness of hormonal contraceptives include:
- barbiturates
- bosentan
- carbamazepine
- felbamate
- griseofulvin
- oxcarbazepine
- phenytoin
- rifampin
- St. John’s wort
- topiramate
HIV protease inhibitors and non-nucleoside reverse transcriptase inhibitors: Significant changes (increase or decrease) in the plasma levels of progestin have been noted in some cases of co-administration of HIV protease inhibitors. Significant changes (increase or decrease) in the plasma levels of the progestin have been noted in some cases of co-administration with non-nucleoside reverse transcriptase inhibitors.
Antibiotics: There have been reports of pregnancy while taking hormonal contraceptives and antibiotics, but clinical pharmacokinetic studies have not shown consistent effects of antibiotics on plasma concentrations of synthetic steroids.
Consult the labeling of all concurrently-used drugs to obtain further information about interactions with hormonal contraceptives or the potential for enzyme alterations.
Laboratory Test Interactions
The pathologist should be advised of progestin therapy when relevant specimens are submitted.
The following laboratory tests may be affected by progestins including medroxyprogesterone acetate injectable suspension:
- Plasma and urinary steroid levels are decreased (e.g., progesterone, estradiol, pregnanediol, testosterone, cortisol).
- Gonadotropin levels are decreased.
- Sex-hormone-binding-globulin concentrations are decreased.
- Protein-bound iodine and butanol extractable protein-bound iodine may increase. T 3-uptake values may decrease.
- Coagulation test values for prothrombin (Factor II), and Factors VII, VIII, IX, and X may increase.
- Sulfobromophthalein and other liver function test values may be increased.
- The effects of medroxyprogesterone acetate on lipid metabolism are inconsistent. Both increases and decreases in total cholesterol, triglycerides, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol have been observed in studies.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): X
Medroxyprogesterone acetate injectable suspension should not be administered during pregnancy.
Pregnancy Category (AUS): D
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Medroxyprogesterone acetate (injection) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Medroxyprogesterone acetate (injection) during labor and delivery.
Nursing Mothers
Detectable amounts of drug have been identified in the milk of mothers receiving medroxyprogesterone acetate injectable suspension.
Pediatric Use
Medroxyprogesterone acetate injectable suspension is not indicated before menarche. Use of medroxyprogesterone acetate injectable suspension is associated with significant loss of BMD. This loss of BMD is of particular concern during adolescence and early adulthood, a critical period of bone accretion. In adolescents, interpretation of BMD results should take into account patient age and skeletal maturity. It is unknown if use of medroxyprogesterone acetate injectable suspension by younger women will reduce peak bone mass and increase the risk of osteoporotic fractures in later life. Other than concerns about loss of BMD, the safety and effectiveness are expected to be the same for postmenarchal adolescents and adult women.
Geriatic Use
This product has not been studied in post-menopausal women and is not indicated in this population.
Gender
There is no FDA guidance on the use of Medroxyprogesterone acetate (injection) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Medroxyprogesterone acetate (injection) with respect to specific racial populations.
Renal Impairment
The effect of renal impairment on medroxyprogesterone acetate injectable suspension pharmacokinetics has not been studied.
Hepatic Impairment
The effect of hepatic impairment on medroxyprogesterone acetate injectable suspension pharmacokinetics has not been studied. Medroxyprogesterone acetate injectable suspension should not be used by women with significant liver disease and should be discontinued if jaundice or disturbances of liver function occur.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Medroxyprogesterone acetate (injection) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Medroxyprogesterone acetate (injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
- intramuscular
Monitoring
A woman who is taking hormonal contraceptive should have a yearly visit with her healthcare provider for a blood pressure check and for other indicated healthcare.
IV Compatibility
There is limited information regarding IV Compatibility of Medroxyprogesterone acetate (injection) in the drug label.
Overdosage
There is limited information regarding Chronic Overdose of Medroxyprogesterone acetate (injection) in the drug label.
Pharmacology
Mechanism of Action
Medroxyprogesterone acetate injectable suspension, when administered at the recommended dose to women every 3 months, inhibits the secretion of gonadotropins which, in turn, prevents follicular maturation and ovulation and results in endometrial thinning. These actions produce its contraceptive effect.
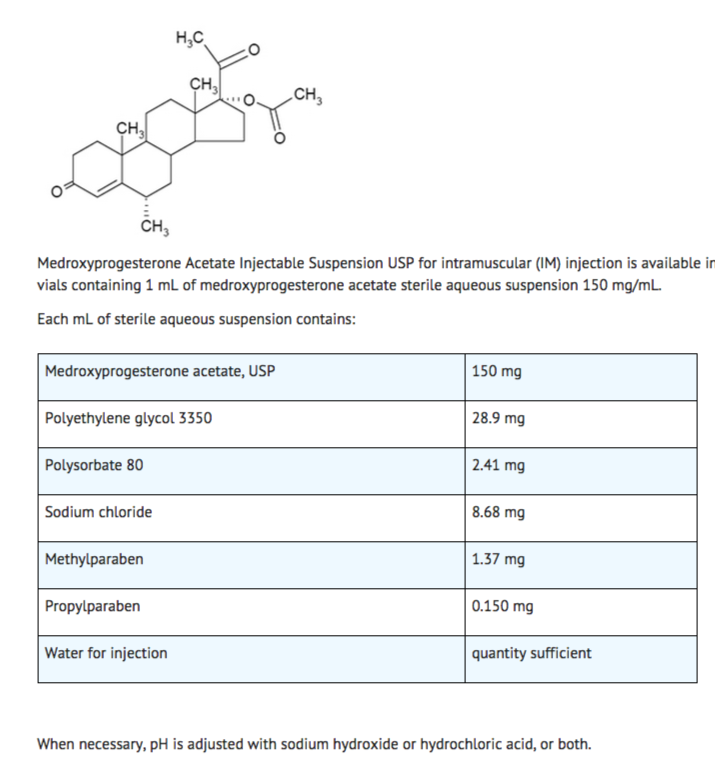
Structure
Medroxyprogesterone Acetate Injectable Suspension USP, a contraceptive injection, contains medroxyprogesterone acetate, USP a derivative of progesterone, as its active ingredient. Medroxyprogesterone acetate is active by the parenteral and oral routes of administration. It is a white to off-white, odorless, fine powder that is stable in air and that melts between 200°C and 210°C. It is freely soluble in chloroform, soluble in acetone and dioxane, sparingly soluble in alcohol and methanol, slightly soluble in ether, and insoluble in water.
The chemical name for medroxyprogesterone acetate, USP is pregn-4-ene-3, 20-dione,17-(acetyloxy)-6-methyl-, (6α)-. The structural formula is as follows:
Pharmacodynamics
No specific pharmacodynamic studies were conducted with medroxyprogesterone acetate injectable suspension.
Pharmacokinetics
Absorption
Following a single 150 mg IM dose of medroxyprogesterone acetate injectable suspension in eight women between the ages of 28 and 36 years old, medroxyprogesterone acetate concentrations, measured by an extracted radioimmunoassay procedure, increase for approximately 3 weeks to reach peak plasma concentrations of 1 to 7 ng/mL.
Distribution
Plasma protein binding of MPA averages 86%. MPA binding occurs primarily to serum albumin. No binding of MPA occurs with sex-hormone-binding globulin (SHBG).
Metabolism
MPA is extensively metabolized in the liver by P450 enzymes. Its metabolism primarily involves ring A and/or side-chain reduction, loss of the acetyl group, hydroxylation in the 2-, 6-, and 21-positions or a combination of these positions, resulting in more than 10 metabolites.
Excretion
The concentrations of medroxyprogesterone acetate decrease exponentially until they become undetectable (< 100 pg/mL) between 120 to 200 days following injection. Using an unextracted radioimmunoassay procedure for the assay of medroxyprogesterone acetate in serum, the apparent half-life for medroxyprogesterone acetate following IM administration of medroxyprogesterone acetate injectable suspension is approximately 50 days. Most medroxyprogesterone acetate metabolites are excreted in the urine as glucuronide conjugates with only minor amounts excreted as sulfates.
Specific Populations
The effect of hepatic and/or renal impairment on the pharmacokinetics of medroxyprogesterone acetate injectable suspension is unknown.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Medroxyprogesterone acetate (injection) in the drug label.
Clinical Studies
1 Contraception
In five clinical studies using medroxyprogesterone acetate injectable suspension, the 12-month failure rate for the group of women treated with medroxyprogesterone acetate injectable suspension was zero (no pregnancies reported) to 0.7 by Life-Table method. The effectiveness of medroxyprogesterone acetate injectable suspension is dependent on the patient returning every 3 months (13 weeks) for reinjection.
2 Bone Mineral Density (BMD) Changes in Adult Women
In a controlled, clinical study, adult women using medroxyprogesterone acetate injectable suspension for up to 5 years showed spine and hip BMD mean decreases of 5 to 6%, compared to no significant change in BMD in the control group. The decline in BMD was more pronounced during the first two years of use, with smaller declines in subsequent years. Mean changes in lumbar spine BMD of -2.86%, -4.11%, -4.89%, -4.93% and -5.38% after 1, 2, 3, 4, and 5 years, respectively, were observed. Mean decreases in BMD of the total hip and femoral neck were similar.
After stopping use of medroxyprogesterone acetate injectable suspension (150 mg), there was partial recovery of BMD toward baseline values during the 2-year post-therapy period. Longer duration of treatment was associated with less complete recovery during this 2-year period following the last injection. Table 4 shows the change in BMD in women after 5 years of treatment with medroxyprogesterone acetate injectable suspension and in women in a control group, as well as the extent of recovery of BMD for the subset of the women for whom 2-year post treatment data were available.

3 Bone Mineral Density Changes in Adolescent Females (12 to 18 years of age)
The impact of medroxyprogesterone acetate injectable suspension (150 mg) use for up to 240 weeks (4.6 years) was evaluated in an open-label non-randomized clinical study in 389 adolescent females (12 to18 years). Use of medroxyprogesterone acetate injectable suspension was associated with a significant decline from baseline in BMD.
Partway through the trial, drug administration was stopped (at 120 weeks). The mean number of injections per medroxyprogesterone acetate injectable suspension user was 9.3. The decline in BMD at total hip and femoral neck was greater with longer duration of use (see Table 5). The mean decrease in BMD at 240 weeks was more pronounced at total hip (-6.4%) and femoral neck (-5.4%) compared to lumbar spine (-2.1%).
In general, adolescents increase bone density during the period of growth following menarche, as seen in the untreated cohort. However, the two cohorts were not matched at baseline for age, gynecologic age, race, BMD and other factors that influence the rate of acquisition of bone mineral density.
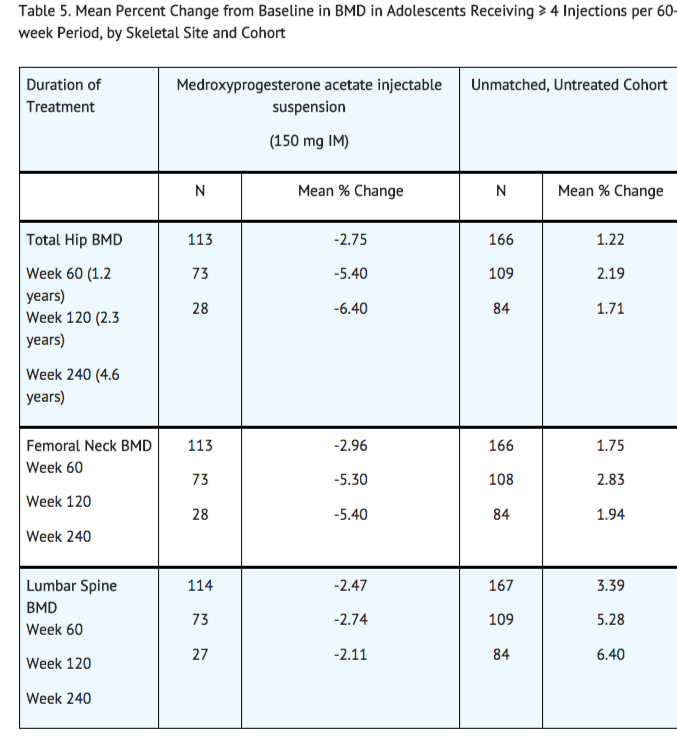
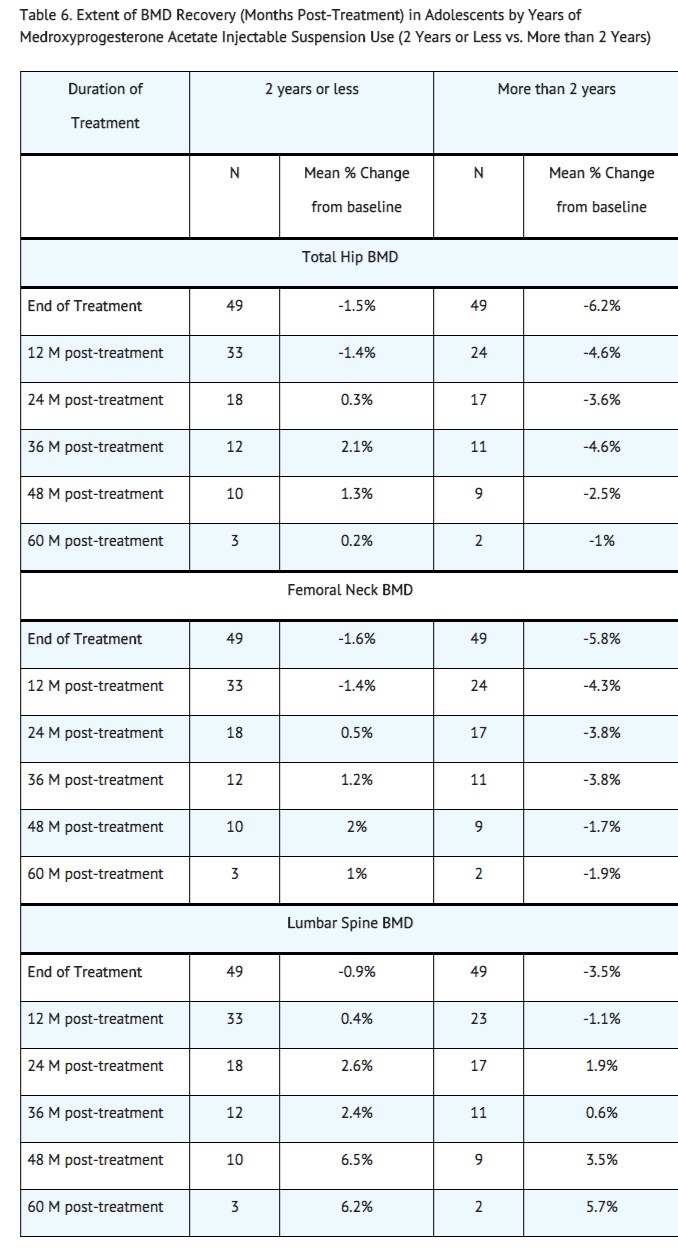
BMD recovery post-treatment in adolescent women
Longer duration of treatment and smoking were associated with less recovery of BMD following the last injection of medroxyprogesterone acetate injectable suspension. Table 6 shows the extent of recovery of BMD up to 60 months post-treatment for adolescent women who received medroxyprogesterone acetate injectable suspension for two years or less compared to more than two years. Post-treatment follow-up showed that, in women treated for more than two years, only lumbar spine BMD recovered to baseline levels after treatment was discontinued. Subjects treated with medroxyprogesterone acetate injectable suspension for more than two years did not recover to their baseline BMD level at femoral neck and total hip even up to 60 months post-treatment. Adolescent women in the untreated cohort gained BMD throughout the trial period (data not shown).
4 Relationship of Fracture Incidence to use of DMPA 150 mg IM or non-use by Women of Reproductive Age
A retrospective cohort study to assess the association between DMPA injection and the incidence of bone fractures was conducted in 312,395 female contraceptive users in the UK. The incidence rates of fracture were compared between DMPA users and contraceptive users who had no recorded use of DMPA. The Incident Rate Ratio (IRR) for any fracture during the follow-up period (mean = 5.5 years) was 1.41 (95% CI 1.35, 1.47). It is not known if this is due to DMPA use or to other related lifestyle factors that have a bearing on fracture rate.
In the study, when cumulative exposure to DMPA was calculated, the fracture rate in users who received fewer than 8 injections was higher than that in women who received 8 or more injections. However, it is not clear that cumulative exposure, which may include periods of intermittent use separated by periods of non-use, is a useful measure of risk, as compared to exposure measures based on continuous use.
There were very few osteoporotic fractures (fracture sites known to be related to low BMD) in the study overall, and the incidence of osteoporotic fractures was not found to be higher in DMPA users compared to non-users. Importantly, this study could not determine whether use of DMPA has an effect on fracture rate later in life.
How Supplied
Storage
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Images
Drug Images
{{#ask: Page Name::Medroxyprogesterone acetate (injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
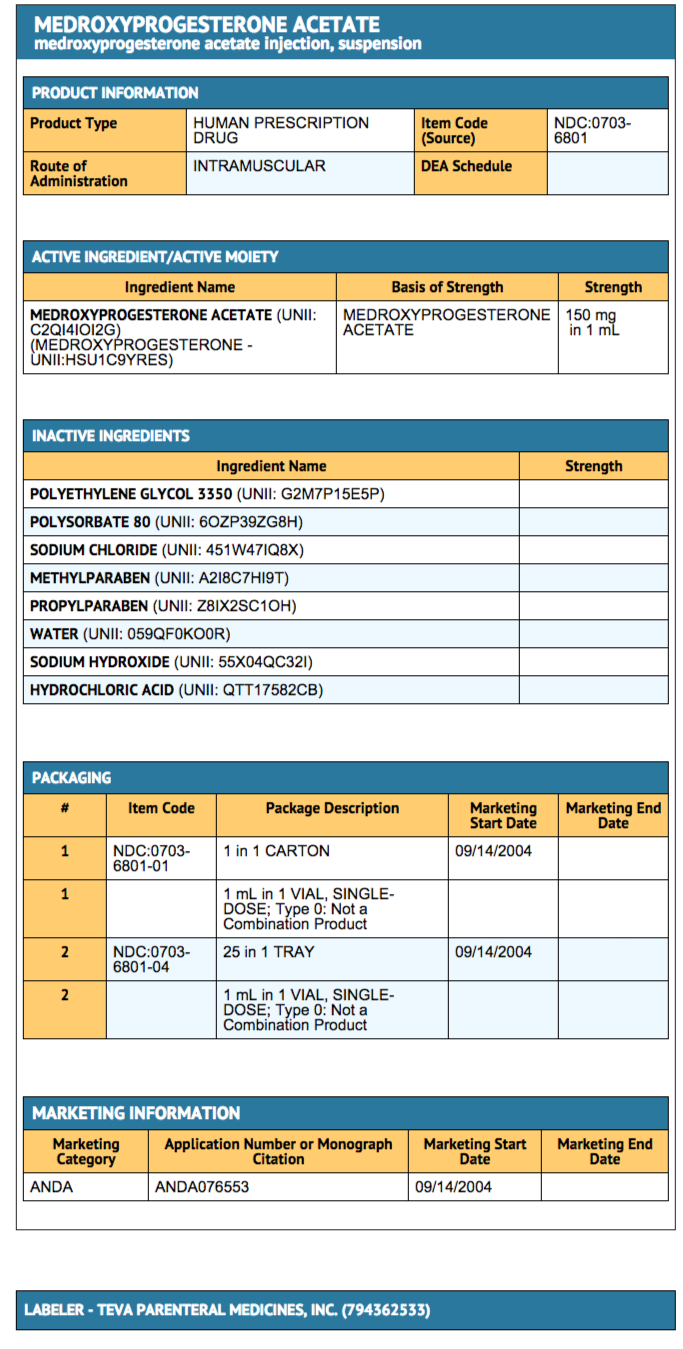
{{#ask: Label Page::Medroxyprogesterone acetate (injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise patients at the beginning of treatment that their menstrual cycle may be disrupted and that irregular and unpredictable bleeding or spotting results, and that this usually decreases to the point of amenorrhea as treatment with medroxyprogesterone acetate injectable suspension continues, without other therapy being required.
- Counsel patients about the possible increased risk of breast cancer in women who use medroxyprogesterone acetate injectable suspension.
- Counsel patients that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
- Counsel patients on Warnings and Precautions associated with use of medroxyprogesterone acetate injectable suspension.
- Counsel patients to use a back-up method or alternative method of contraception when enzyme inducers are used with medroxyprogesterone acetate injectable suspension.
Precautions with Alcohol
- Alcohol-Medroxyprogesterone acetate (injection) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- MEDROXYPROGESTERONE ACETATE®[1]
Look-Alike Drug Names
- Depo-Provera® — Depo-subQ provera 104®[2]
- medroxyPROGESTERone ® — methotrexate®[2]
- medroxyPROGESTERone® — methylPREDNISolone®[2]
- medroxyPROGESTERone® — methylTESTOSTERone®[2]
- Provera® — Proscar®[2]
- Provera® — Prozac®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "MEDROXYPROGESTERONE ACETATE- medroxyprogesterone acetate injection, suspension".
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 "http://www.ismp.org". External link in
|title=(help)