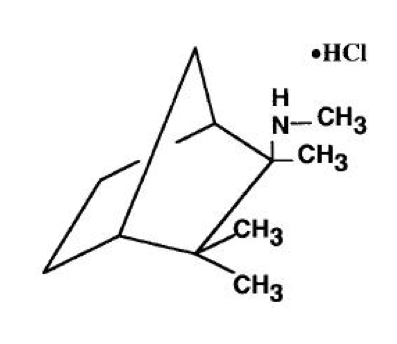
Mecamylamine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gerald Chi
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Mecamylamine is a autonomic ganglionic blocker that is FDA approved for the {{{indicationType}}} of moderately severe to severe essential hypertension and uncomplicated cases of malignant hypertension. Common adverse reactions include orthostatic hypotension, dizziness, lightheadedness, and fainting.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Essential Hypertension and Malignant Hypertension
- Therapy is usually started with one 2.5 mg tablet of Mecamylamine HCl twice a day. This initial dosage should be modified by increments of one 2.5 mg tablet at intervals of not less than 2 days until the desired blood pressure response occurs (the criterion being a dosage just under that which causes signs of mild postural hypotension).
The average total daily dosage of Mecamylamine HCl is 25 mg, usually in three divided doses. However, as little as 2.5 mg daily may be sufficient to control hypertension in some patients. A range of two to four or even more doses may be required in severe cases when smooth control is difficult to obtain. In severe or urgent cases, larger increments at smaller intervals may be needed. Partial tolerance may develop in certain patients, requiring an increase in the daily dosage of Mecamylamine HCl.
- Administration of Mecamylamine HCl after meals may cause a more gradual absorption and smoother control of excessively high blood pressure. The timing of doses in relation to meals should be consistent. Since the blood pressure response to antihypertensive drugs is increased in the early morning, the larger dose should be given at noontime and perhaps in the evening. The morning dose, as a rule, should be relatively small and in some instances may even be omitted.
- The initial regulation of dosage should be determined by blood pressure readings in the erect position at the time of maximal effect of the drug, as well as by other signs and symptoms of orthostatic hypotension.
- The effective maintenance dosage should be regulated by blood pressure readings in the erect position and by limitation of dosage to that which causes slight faintness or dizziness in this position. If the patient or a relative can use a sphygmomanometer, instructions may be given to reduce or omit a dose if readings fall below a designated level or if faintness or lightheadedness occurs. However, no change should be instituted without the knowledge of the physician.
Close supervision and education of the patient, as well as critical adjustment of dosage, are essential to successful therapy.
- Other Antihypertensive Agents
- When Mecamylamine HCl is given with other antihypertensive drugs, the dosage of these other agents, as well as that of Mecamylamine HCl, should be reduced to avoid excessive hypotension. However, thiazides should be continued in their usual dosage, while that of Mecamylamine HCl is decreased by at least 50 percent.
- Dosing Information
- Dosage
Condition2
- Dosing Information
- Dosage
Condition3
- Dosing Information
- Dosage
Condition4
- Dosing Information
- Dosage
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Mecamylamine in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Mecamylamine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Mecamylamine in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Mecamylamine in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Mecamylamine in pediatric patients.
Contraindications
- Condition1
Warnings
- Description
Precautions
- Description
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Mecamylamine in the drug label.
Central Nervous System
Cardiovascular
Respiratory
Gastrointestinal
Hypersensitivity
Miscellaneous
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Mecamylamine in the drug label.
Central Nervous System
Cardiovascular
Respiratory
Gastrointestinal
Hypersensitivity
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Mecamylamine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Mecamylamine during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Mecamylamine with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Mecamylamine with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Mecamylamine with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Mecamylamine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Mecamylamine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Mecamylamine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Mecamylamine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Mecamylamine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Mecamylamine in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Mecamylamine in the drug label.
Condition1
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Mecamylamine in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Mecamylamine in the drug label.
Pharmacology
| |
Mecamylamine
| |
| Systematic (IUPAC) name | |
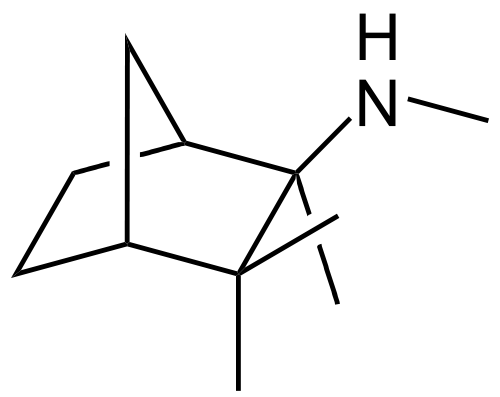
| (1S,2R,4R)-N,2,3,3-tetramethylbicyclo[2.2.1]heptan-2-amine | |
| Identifiers | |
| CAS number | |
| ATC code | C02 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 167.291 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | 40% |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
Template:Unicode Prescription only |
| Routes | Oral |
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Mecamylamine in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Mecamylamine in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Mecamylamine in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Mecamylamine in the drug label.
Condition1
- Description
How Supplied
- VecamylTM [Mecamylamine HCl Tablet USP] are slightly yellow, round, compressed tablets, coded MP on one side and 2.5 on the other side. They are supplied as follows:
- NDC 0722-7183-01 in bottles of 100.
- Storage
- Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F)
Storage
There is limited information regarding Mecamylamine Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Mecamylamine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Mecamylamine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Mecamylamine HCl may cause dizziness, lightheadedness, or fainting, especially when rising from a lying or sitting position. This effect may be increased by alcoholic beverages, exercise, or during hot weather. Getting up slowly may help alleviate such a reaction.
Precautions with Alcohol
- The action of Mecamylamine may be potentiated by alcohol.
- Mecamylamine may cause dizziness, lightheadedness, or fainting, especially when rising from a lying or sitting position. This effect may be increased by alcoholic beverages.
Brand Names
- Vecamyl®[1]
Look-Alike Drug Names
- N/A[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "VECAMYL (mecamylamine hydrochloride) tablet".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Mecamylamine |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Mecamylamine |Label Name=Mecamylamine02.png
}}
{{#subobject:
|Label Page=Mecamylamine |Label Name=Mecamylamine03.png
}}