Marfan's syndrome: Difference between revisions
| Line 235: | Line 235: | ||
Molecular diagnostics, namely DNA sequencing can be extremely informative for the diagnosis of Marfan syndrome. A 2008 study showed that while only 79% of known probands could be diagnosed with Marfan syndrome using clinical criteria, 90% of these individuals could be diagnosed using the international criteria when sequencing data was added. In children, this figure leapt from 56% to 85% with sequencing data. The increased diagnostic sensitivity conferred by genetic information promises to be especially useful in children who have not developed clinical manifestations, but in whom new pharmacological interventions may be successful. Currently, laboratories offer complete sequencing of the 65 exon FBN1 gene for approximately $1700.00. The high costs of these diagnostics have delayed the widespread use of molecular diagnostics in the approach to patients suspected of Marfan syndrome. Increasingly less expensive sequencing technologies promise to increase reliance on individual genetic data for diagnosis in the future. | Molecular diagnostics, namely DNA sequencing can be extremely informative for the diagnosis of Marfan syndrome. A 2008 study showed that while only 79% of known probands could be diagnosed with Marfan syndrome using clinical criteria, 90% of these individuals could be diagnosed using the international criteria when sequencing data was added. In children, this figure leapt from 56% to 85% with sequencing data. The increased diagnostic sensitivity conferred by genetic information promises to be especially useful in children who have not developed clinical manifestations, but in whom new pharmacological interventions may be successful. Currently, laboratories offer complete sequencing of the 65 exon FBN1 gene for approximately $1700.00. The high costs of these diagnostics have delayed the widespread use of molecular diagnostics in the approach to patients suspected of Marfan syndrome. Increasingly less expensive sequencing technologies promise to increase reliance on individual genetic data for diagnosis in the future. | ||
{| style="padding:0.3em; float:left; margin-left:5px; border:1px solid #A3B1BF;" | |||
|- | |||
|[[Image:Marfan pic1.jpeg|150px]] | |||
|[[Image:Marfan pic2.jpeg|150px]] | |||
|- | |||
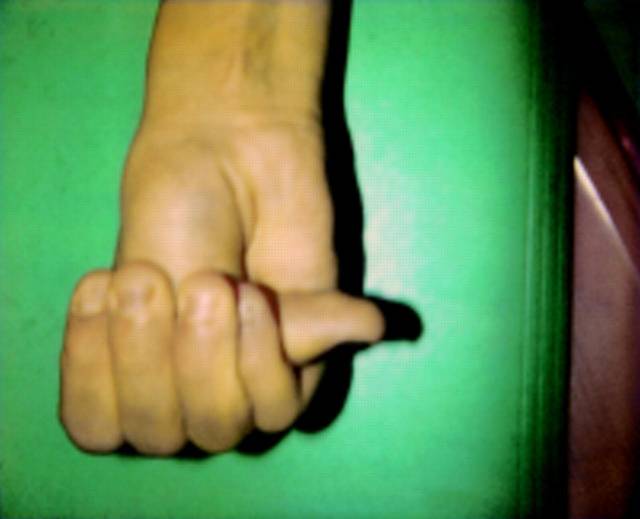
|<small> '''Image A:''' Skeletal exam of Marfan syndrome. Thumb sign, positive if the thumb protrudes from the clenched fist.</small> | |||
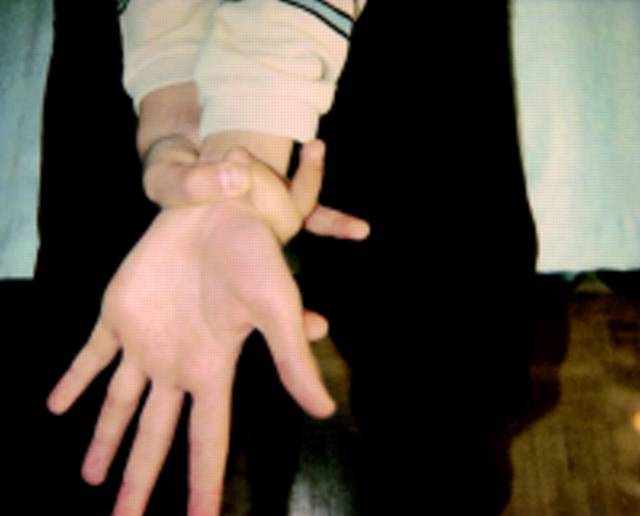
|<small>'''Image B:''' Skeletal exam of Marfan syndrome. Wrist Sign, positive if thumb and first phalange can overlap when encircling wrist.</small> | |||
<ref name="pmid11711445">{{cite journal |author=Cocco G |title=Images in cardiology: The "thumb and wrist sign" in Marfan syndrome |journal=[[Heart (British Cardiac Society)]] |volume=86 |issue=6 |pages=602 |year=2001 |month=December |pmid=11711445 |pmc=1730018 |doi= |url=http://heart.bmj.com/cgi/pmidlookup?view=long&pmid=11711445 |issn= |accessdate=2010-12-22}}</ref> | |||
|} | |||
{{clr}} | |||
==Management== | ==Management== | ||
Revision as of 17:11, 22 December 2010
| Marfan syndrome | |
 | |
|---|---|
| ICD-10 | Q87.4 |
| ICD-9 | 759.82 |
| OMIM | 154700 |
| DiseasesDB | 7845 |
| MedlinePlus | 000418 |
| eMedicine | ped/1372 orthoped/414 |
| MeSH | C17.300.500 |
|
WikiDoc Resources for Marfan's syndrome |
|
Articles |
|---|
|
Most recent articles on Marfan's syndrome Most cited articles on Marfan's syndrome |
|
Media |
|
Powerpoint slides on Marfan's syndrome |
|
Evidence Based Medicine |
|
Cochrane Collaboration on Marfan's syndrome |
|
Clinical Trials |
|
Ongoing Trials on Marfan's syndrome at Clinical Trials.gov Trial results on Marfan's syndrome Clinical Trials on Marfan's syndrome at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Marfan's syndrome NICE Guidance on Marfan's syndrome
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Marfan's syndrome Discussion groups on Marfan's syndrome Patient Handouts on Marfan's syndrome Directions to Hospitals Treating Marfan's syndrome Risk calculators and risk factors for Marfan's syndrome
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Marfan's syndrome |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editors-In-Chief: William James Gibson, C. Michael Gibson, M.S., M.D.
Associate Editor-In-Chief: Cafer Zorkun, M.D., Ph.D. [1]
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [2] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
Marfan syndrome (or Marfan's syndrome) is a connective tissue disorder most often caused by defects in the Fibrillin-1 gene (FBN1). Patients with Marfan's syndrome are at significant risk of skeletal, cardiovascular and ocular complications. People with Marfan's are typically tall, with long limbs and long thin fingers.
Background
In 1896, French pediatrician Antoine-Bernard Jean Marfan described a five year old girl, Gabrielle P, with skeletal features characteristic of Marfan Syndrome, pieds d’aragne (French, spider feet) and dolichostenomalie (French, longheadedness meaning long limbs). In 1902, Emile Charles Achard described a similar syndrome, reporting scoliosis and arachnodactyly (abnormally long and slender fingers) as essential features [1]. Salle contributed the observation in 1912 that patients with arachnodactyly had thickened mitral leaflets, ocular abnormalities and increase in eosinophilic cells in the pituitary [2]. The observation that ectopic lens was associated with other symptoms was first made by Boerger in 1914 . Weve established the autosomal dominant inheritance of the disease, still known as arachnodactyly, in 1931. Weve postulated that the syndrome arose from a defect in mesenchymal tissue and thus designated the syndrome dystrophia mesodermalis congenita typus Marfanis. Association of the syndrome with aortic dilation and dissection, the major causes of mortality in individuals with Marfan Syndrome were identified in 1943 by RW Baer et al. as well as Etter and Glover. Harry C Deitz finally established the molecular basis of Marfan Syndrome in his landmark 1991 Nature paper, showing that mutations in the FBN1 gene are responsible for the disease [3].
Pathophysiology
Marfan syndrome has been linked to a defect in the FBN1 gene on chromosome 15,[4] which encodes a glycoprotein called fibrillin-1. Fibrillin is essential for the formation of the elastic fibers found in connective tissue, as it provides the scaffolding for tropoelastin.[5] Elastic fibers are found throughout the body but are particularly abundant in the aorta, ligaments and the ciliary zonules of the eye, consequently these areas are among the worst affected. Without the structural support provided by fibrillin many connective tissues are weakened, which can have severe consequences for support and stability.
Marfan syndrome is inherited as a dominant trait. In so far as the pattern of inheritance is dominant, people who have inherit just one affected FBN1 gene from either parent will develop Marfan syndrome. This expression of the syndrome can range from mild to severe.
A related disease has been found in mice, and the study of mouse fibrillin synthesis and secretion, and connective tissue formation, has begun to further our understanding of Marfan syndrome in humans. It has been found that simply reducing the level of normal fibrillin-1 causes a Marfan-related disease in mice.[6]
High levels of Transforming growth factor beta (TGFβ) are associated with inflammation and also play an important role in Marfan syndrome. Ordinarily, Fibrillin-1 binds TGFβ and inactivates it. In Marfan syndrome, reduced levels of fibrillin-1 allow activated TGFβ to damage the lungs and heart. Researchers now believe that the inflammatory effects of TGF-β, on the lungs, heart valves, and aorta weaken the connective tissues and cause the features of Marfan syndrome. In so far as angiotensin II receptor blockers (ARBs) reduce TGF-β, these agents have been administered to young Marfan syndrome patients, and the expansion of the aorta was indeed reduced.[7]
A defect in the gene TGFβR2 on chromosome 3, a receptor protein of TGFβ, has also been related to Marfan syndrome.[8] Marfan syndrome can often be confused with Loeys-Dietz syndrome, a similar connective tissue disorder resulting from mutations in the TGFβ receptor genes TGFβR1 and TGFβR2.[9]
Differential Diagnosis
The following disorders have similar signs and symptoms of Marfan syndrome:
- Congenital Contractural Arachnodactyly (CCA) or Beals Syndrome
- Ehlers-Danlos syndrome
- Homocystinuria
- Loeys-Dietz syndrome
- MASS phenotype
- Stickler syndrome
Etymology
In 1896, French pediatrician Antoine-Bernard Jean Marfan described a five year old girl, Gabrielle P, with skeletal features characteristic of Marfan Syndrome1, pieds d’aragne (French, spider feet) and dolichostenomalie (French, longheadedness meaning long limbs). In 1902, Emile Charles Achard described a similar syndrome, reporting scoliosis and arachnodactyly (abnormally long and slender fingers) as essential features2. Salle contributed the observation in 1912 that patients with arachnodactyly had thickened mitral leaflets, ocular abnormalities and increase in eosinophilic cells in the pituitary3,4. The observation that ectopic lens was associated with other symptoms was first made by Boerger in 19145 . Weve established the autosomal dominant inheritance of the disease, still known as arachnodactyly, in 19316. Weve postulated that the syndrome arose from a defect in mesenchymal tissue and thus designated the syndrome dystrophia mesodermalis congenita typus Marfanis. Association of the syndrome with aortic dilation and dissection, the major causes of mortality in individuals with Marfan Syndrome were identified in 1943 by RW Baer et al. as well as Etter and Glover7,8. Harry C Deitz finally established the molecular basis of Marfan Syndrome in his landmark 1991 Nature paper, showing that dysregulation of TGF-beta signaling is responsible for the observed manifestations9.
Epidemiology
Marfan syndrome affects males and females equally,[10] and the mutation shows no geographical bias. Estimates indicate that approximately 60 000 (1 in 5000, or 0.02% of the population)[10] to 200 000[11] Americans have Marfan syndrome. Each parent with the condition has a 50% chance of passing it on to a child due to its autosomal dominant nature. Most individuals with Marfan syndrome have another affected family member, but approximately 15-30% of all cases are due to de novo genetic mutations[5] — such spontaneous mutations occur in about 1 in 20 000 births. Marfan syndrome is also an example of dominant negative mutation and haploinsufficiency.[12][13] It is associated with variable expressivity. Incomplete penetrance, has not been definitively documented.
The prevalence of Marfan syndrome is 1 case per 3000 to 5000 individuals or .033 % (upper estimate) [14]. Neither location nor ethnicity appear to impact this statistic. Populations of certain athletes such as basketball and volleyball players have been shown to have an increased incidence of Marfan syndrome (~0.5%) [15], perhaps due to skeletal abnormalities associated with the syndrome. While patients now have nearly normal life expectancies, in previous decades, patients’ life expectancies were significantly shortened by the risks of aortic dissection, valvular failure and congestive heart failure. Together, these cardiovascular complications accounted for 90% of the mortality associated with Marfan syndrome such that in the 1970s, an affected individual would be expected to live only two-thirds as long as his unaffected counterparts [16].
Related disorders
The following conditions that can result from having Marfan's syndrome and may also occur in people without any known underlying disorder. what leads doctors to a diagnosis of marfan syndrome is family history and a combination of major and minor indicators of the disorder that occur in one individual which is a rare manifestation in general population. Example: four skeletal signs with one or more signs in another body system such as ocular and cardiovascular in one individual.
- Aortic aneurysm or dilatation
- Arachnodactyly
- Bicuspid aortic valve
- Cysts
- Craniosynostosis
- Cystic medial necrosis
- Dural ectasia
- Ectopia lentis
- Flat feet
- Gigantism
- Glaucoma
- Hernias
- Hypermobility of the joints
- Malocclusion
- Mitral valve prolapse
- Myopia
- Obstructive lung disease
- Osteoarthritis
- Pectus carinatum or excavatum
- Pneumothorax
- Retinal detachment
- Scoliosis
- Sleep apnea
- Stretch marks
Symptoms
There are no signs or symptoms that are unique to Marfan syndrome. It is usually a single apparent sign or symptom that leads doctors to look for others and eventually to diagnose the syndrome, which affects connective tissue in diverse organs and systems. Even affected individuals in the same family might exhibit various combinations and severities of symptoms.
Skeletal system
The most readily visible signs are associated with the skeletal system. Many individuals with Marfan Syndrome grow to above average height. Some have long slender limbs with fingers and toes that are also abnormally long and slender (arachnodactyly). An individual's arms may be disproportionately long. In addition to affecting height and limb proportions, Marfan syndrome can produce other skeletal signs. Abnormal curvature of the spine (scoliosis) is common, as is abnormal indentation (pectus excavatum) or protrusion (pectus carinatum) of the sternum. Other signs include abnormal joint flexibility, a high palate, malocclusions, flat feet, stooped shoulders, and unexplained stretch marks on the skin. Some people with Marfans have speech disorders resulting from symptomatic high palates and small jaws.
Eyes
Marfan syndrome can also seriously affect the eyes and vision. Nearsightedness and astigmatism are common, but farsightedness can also result. [17]
Subluxation (dislocation) of the crystalline lens in one or both eyes (ectopia lentis) (in 80% of patients) also occurs and may be detected by an ophthalmologist or optometrist using a slit-lamp biomicroscope. [17]
In Marfan's the dislocation is typically superotemporal whereas in the similar condition homocystinuria, the dislocation is inferonasal.[17]
Sometimes eye problems appear only after the weakening of connective tissue has caused detachment of the retina.[17] Early onset glaucoma can be another related problem.
Cardiovascular system
The most serious conditions associated with Marfan syndrome involve the cardiovascular system. Undue fatigue, shortness of breath, heart palpitations, racing heartbeats, or pain in the left chest, back, shoulder, or arm, can bring an individual into the doctor's office. A heart murmur heard on a stethoscope, an abnormal reading on an electrocardiogram, or symptoms of angina can lead a doctor to order an echocardiogram. This can reveal signs of leakage or prolapse of the mitral or aortic valves that control the flow of blood through the heart. (See mitral valve prolapse.) However, the major sign that would lead a doctor to consider an underlying condition is a dilated aorta or an aortic aneurysm. Sometimes, no heart problems are apparent until the weakening of the connective tissue in the ascending aorta causes an aortic aneurysm or even aortic dissection.
Because of the underlying connective tissue abnormalities that cause Marfan syndrome, there is an increased incidence of dehiscence of prosthetic mitral valve.[18] Care should be taken to attempt repair of damaged heart valves rather than replacement.
During pregnancy, even in the absence of preconceived cardiovascular abnormality, women with Marfan syndrome are at significant risk of acute aortic dissection, which can be lethal if untreated. For this reason, women with Marfan syndrome should receive a thorough assessment prior to conception, and echocardiography should be performed every 6-10 weeks during pregnancy, to assess the aortic root diameter. Most women however tolerate pregnancy well and safe vaginal delivery is possible.[19]
- A typical aortic root in Marfan's syndrome.
<googlevideo>-760162053984535443&hl=en</googlevideo>
Lungs
Marfan syndrome is a risk factor for spontaneous pneumothorax. In spontaneous unilateral pneumothorax, air escapes from a lung and occupies the pleural space between the chest wall and a lung. The lung becomes partially compressed or collapsed. This can cause pain, shortness of breath, cyanosis, and, if not treated, death. Marfan syndrome has also been associated with sleep apnea and idiopathic obstructive lung disease.
Central nervous system
Another condition that can reduce the quality of life for an individual, though not life-threatening, is dural ectasia, the weakening of the connective tissue of the dural sac, the membrane that encases the spinal cord.
Dural ectasia can be present for a long time without producing any noticeable symptoms. Symptoms that can occur are lower back pain, leg pain, abdominal pain, other neurological symptoms in the lower extremities, or headaches. Such symptoms usually diminish when the individual lies flat on his or her back.
These types of symptoms might lead a doctor to order an X-ray of the lower spine. Dural ectasia is usually not visible on an X-ray in the early phases. A worsening of symptoms and the lack of finding any other cause should eventually lead a doctor to order an upright MRI of the lower spine.
Dural ectasia that has progressed to the point of causing these symptoms would appear in an upright MRI image as a dilated pouch that is wearing away at the lumbar vertebrae.[17] Other spinal issues associated with Marfan include degenerative disk disease and spinal cysts.
Diagnosis
Several standards of diagnostic criteria for Marfan syndrome have been proposed. In 1986, the Berlin nosology was established which represented a consensus on clinical features diagnostic of Marfan syndrome with an emphasis on skeletal features [20]. Advances in molecular testing and the realization that many individuals diagnosed with Marfan syndrome according to the Berlin nosology did not have mutations in the FBN1 gene, led to the establishment of the Ghent nosology in1996, a new set of criteria with stricter diagnostic requirements [21]. The Ghent nosology remains the current standard for diagnosis, although a revised version of the guidelines was published in 201015. The criteria are divided into major and minor manifestations which have allowed physicians to correctly diagnose 95% of patients as confirmed by molecular analysis of the FBN1 gene [22]. The new criteria establish aortic root aneurysm and ectopia lentis as the principal clinical features of the disease and stress cardiovascular manifestations.
The major criteria for diagnosis of Marfan syndrome are ectopia lentis, aortic root dilation/dissection, dural ectasia, or a combination of more than 4 out of 8 major skeletal features (Table 1). In an individual with no known family history of Marfan syndrome and in the absence of any known FBN1 mutations, major involvement of two organs systems (e.g. skeletal, cardiovascular, ocular) and minor involvement of a third system is required for diagnosis. However, if the patient has a known FBN1 mutation or affected relative, major involvement of only one system and minor involvement of another is sufficient for diagnosis (Table 1). Major manifestations of the cardiovascular system include ascending aortic dilation involving the sinuses of valsalva or dissection of the ascending aorta. Ectopia Lentis is the sole major criterion for involvement of the ocular system, and dural ectasia in the lumbosacral region diagnosed by CT or MRI is the criterion for major involvement of the dura. Having a known FBN1 mutation or a relative who independently satisfies criteria for diagnosis satisfies major involvement of family/genetic history. Physical examination for Marfan syndrome requires extensive evaluation of the skeletal system. Patients must fulfill four of the eight following criteria for major involvement of the skeletal system: pectus carinatum, pectus ex cavatum requiring surgery, reduced upper to lower segment ratio, wrist and thumb signs (Figure 1). Minor criteria for the various systems including pulmonary and skin/integument can be found in the supplement.
| Skeletal System | |
|---|---|
| Major Criterion. | Presence of at least 4of the following manifestations.
• pectus carinatum • pectus ex cavatum requiring surgery • reduced upper to lower segment ratio or arm span to height ratio greater than 1.05 • wrist and thumb signs • scoliosis of > 20" or spondylolisthesis • reduced extension at the elbows (< 170") • medial displacementof the medial malleolus causing pes planus protmsio acetabulae of any degree (ascertained on radiographs) |
| Minor criteria. |
• pectus excavatum of moderate severity • joint hypermobility • highly arched palate with crowding of teeth • facial appearance (dolichocephaly, malar hypo- plasia,enophthalmos,retrognathia,down-Slanting palpebral fissures) |
| Ocular System | |
| Major criterion. | • Ectopia lentis |
| Minor criteria. |
• abnormally flat cornea (as measured by keratometry) • increased axial length of globe (as measured by ultrasound) • hypoplastic iris or hypoplastic ciliary muscle causing decreased miosis |
| Cardiovascular System | |
| Major criteria. |
• dilatation of the ascending aorta with or without aortic regurgitation and involving at least the sinuses of Valsalva; or • dissection of the ascending aorta |
| Minor criteria. |
• Mitral valve prolapsed • Mitral valve prolapsed with or without mitral valve regurgitation; • dilatation of the main pulmonary artery, in the absence of valvular or peripheral pulmonic stenosis or any other obvious cause, below the age of 40 years; • calcification of the mitral annulus below the age of 40 years; or dilatation or dissection of the descending thoracic or abdominal aorta below the age of 50 years |
| Pulmonary System | |
| Minor criteria. |
• spontaneous pneumothorax [Hall et al., 19841] • apical blebs (ascertained by chest radiography) |
| Skin and Integument | |
| Minor criteria. |
• striaeatrophicae (stretchmarks) not associated with marked weight changes, pregnancy or repetitive stress • recurrent or incisional herniae |
| Dura | |
| Major criterion. |
• Lumbosacral dural ectasia by CT or MRI |
| Family/Genetic History | |
| Major criteria. | • having a parent, child or sib who meets these diagnostic criteria independently;
• presence of a mutation in FBNl known to cause the Marfan syndrome; • presence of a haplotype around FBNl, inherited by descent, known to be associated with unequivocal- ly diagnosed Marfan syndrome in the family |
Adapted from De Paepe A, Devereux RB, Dietz HC, Hennekam RC, Pyeritz RE. Revised diagnostic criteria for the Marfan syndrome. Am J Med Genet 1996;62:417-26.
Molecular diagnostics, namely DNA sequencing can be extremely informative for the diagnosis of Marfan syndrome. A 2008 study showed that while only 79% of known probands could be diagnosed with Marfan syndrome using clinical criteria, 90% of these individuals could be diagnosed using the international criteria when sequencing data was added. In children, this figure leapt from 56% to 85% with sequencing data. The increased diagnostic sensitivity conferred by genetic information promises to be especially useful in children who have not developed clinical manifestations, but in whom new pharmacological interventions may be successful. Currently, laboratories offer complete sequencing of the 65 exon FBN1 gene for approximately $1700.00. The high costs of these diagnostics have delayed the widespread use of molecular diagnostics in the approach to patients suspected of Marfan syndrome. Increasingly less expensive sequencing technologies promise to increase reliance on individual genetic data for diagnosis in the future.
Management
There is no cure for Marfan syndrome, but life expectancy has increased significantly over the last few decades, and clinical trials are underway for a promising new treatment.[24] The syndrome is treated by addressing each issue as it arises, and, in particular, considering prophylactic medication, even for young children, to slow progression of aortic dilation.
Regular checkups by a cardiologist are needed to monitor the health of the heart valves and the aorta. The goal of treatment is to slow the progression of aortic dilation and damage to heart valves by eliminating arrythmias, minimizing the heart rate, and minimizing blood pressure.
Beta blockers have been used to control arrythmias and slow the heart rate. Other medications might be needed to further minimize blood pressure without slowing the heart rate, such as ACE inhibitors and angiotensin II receptor antagonists, also known as angiontensin receptor blockers (ARBs).
If the dilation of the aorta progresses to a significant diameter aneurysm, causes a dissection or a rupture, or leads to failure of the aortic or other valve, then surgery (possibly a composite aortic valve graft [CAVG] or valve-sparing procedure) becomes necessary.
Although aortic graft surgery (or any vascular surgery) is a serious undertaking it is generally successful if undertaken on an elective basis. Surgery in the setting of acute aortic dissection or rupture is considerably more problematic. Elective aortic valve/graft surgery is usually considered when aortic root diameter reaches 50 millimetres, but each case needs to be specifically evaluated by a qualified cardiologist. New valve-sparing surgical techniques are becoming more common.[25] As Marfan patients live longer, other vascular repairs are becoming more common, e.g. repairs of descending thoractic aortic aneurysms and aneurysms of vessels other than the aorta.
The skeletal and ocular manifestations of Marfan syndrome can also be serious, although not life-threatening. These symptoms are usually treated in the typical manner for the appropriate condition. This can also affect height, arm length, and life span. The Nuss procedure is now being offered to people with Marfan syndrome to correct 'sunken chest' or (pectus excavatum).[26] Because Marfan may cause spinal abnormalities that are asymptomatic, any spinal surgery contemplated on a Marfan patient should only follow detailed imaging and careful surgical planning, regardless of the indication for surgery.
Clinical trials have been conducted of the drug acetazolamide in the treatment of symptoms of dural ectasia. The treatment has demonstrated significant functional improvements in some sufferers.[27] Other medical treatments, as well as physical therapy, are also available.
Treatment of a spontaneous pneumothorax is dependant on the volume of air in the pleural space and the natural progression of the individual's condition. A small pneumothorax might resolve without active treatment in 1 to 2 weeks. Recurrent pneumothoraxes might require chest surgery. Moderately sized pneumothoraxes might need chest drain management for several days in hospital. Large pneumothoraxes are likely to be medical emergencies requiring emergency decompression.
Research in laboratory mice has suggested that the angiotensin II receptor antagonist losartan, which appears to block TGF-beta activity, can slow or halt the formation of aortic aneurysms in Marfan syndrome.[28] A large clinical trial sponsored by the National Institutes of Health comparing the effects of losartan and atenolol on the aortas of Marfan patients is scheduled to begin in early 2007, coordinated by Johns Hopkins.[29]
Genetic counseling and specialized clinics are available at many academic medical centers for affected persons and family members.
References
- ↑ BOYER BE, MARTIN MM (1958). "Marfan's syndrome; report of a case manifesting a giant bone cyst of the mandible and multiple (110) basal cell carcinomata". Plastic and Reconstructive Surgery and the Transplantation Bulletin. 22 (3): 257–63. PMID 13590978. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ BLACK HH, LANDAY LH (1955). "Marfan's syndrome; report on five cases in one family". A.M.A. American Journal of Diseases of Children. 89 (4): 414–20. PMID 14360720. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Dietz HC, Cutting GR, Pyeritz RE, Maslen CL, Sakai LY, Corson GM, Puffenberger EG, Hamosh A, Nanthakumar EJ, Curristin SM (1991). "Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene". Nature. 352 (6333): 337–9. doi:10.1038/352337a0. PMID 1852208. Retrieved 2010-12-22. Unknown parameter
|month=ignored (help) - ↑ McKusick V (1991). "The defect in Marfan syndrome". Nature. 352 (6333): 279–81. PMID 1852198.
- ↑ 5.0 5.1 Cotran. Robbins Pathologic Basis of Disease. Philadelphia: W.B Saunders Company. 0-7216-7335-X. Unknown parameter
|coauthors=ignored (help) - ↑ Lygia Pereira; et al. (1999). "Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1". Proceedings of the National Academy of Sciences. 96 (7): 3819-3823.
- ↑ Pyeritz RE (2008). "A small molecule for a large disease". N. Engl. J. Med. 358 (26): 2829–31. doi:10.1056/NEJMe0804008. PMID 18579819. Unknown parameter
|month=ignored (help) - ↑ Entrez Gene (2007). "TGFBR2 transforming growth factor, beta receptor II" (Entrez gene entry). NCBI.
- ↑ "Related Disorders: Loeys-Dietz". National Marfan Foundation.
- ↑ 10.0 10.1 "The role of heredity and family history". National Marfan Foundation. 1999.
- ↑ "New, Deadly Relative of Marfan's Syndrome Discovered". MedicineNet.com. 2006.
- ↑ Judge, Daniel P. "Evidence for a critical contribution of haploinsufficiency in the complex pathogenesis of Marfan syndrome". The Journal of Clinical Investigation. 114 (2): 172–181. doi:10.1172/JCI200420641. PMID 15254584. Unknown parameter
|coauthors=ignored (help) - ↑ Judge, Daniel P. (2005). "Marfan's syndrome". Lancet. 366 (9501): 1965–76. doi:10.1016/S0140-6736(05)67789-6. Check
|doi=value (help). PMID 16325700. Unknown parameter|coauthors=ignored (help) - ↑ Judge DP, Dietz HC (2005). "Marfan's syndrome". Lancet. 366 (9501): 1965–76. doi:10.1016/S0140-6736(05)67789-6. PMC 1513064. PMID 16325700. Retrieved 2010-12-22. Unknown parameter
|month=ignored (help) - ↑ Kinoshita N, Mimura J, Obayashi C, Katsukawa F, Onishi S, Yamazaki H (2000). "Aortic root dilatation among young competitive athletes: echocardiographic screening of 1929 athletes between 15 and 34 years of age". American Heart Journal. 139 (4): 723–8. PMID 10740158. Retrieved 2010-12-22. Unknown parameter
|month=ignored (help) - ↑ Murdoch JL, Walker BA, Halpern BL, Kuzma JW, McKusick VA (1972). "Life expectancy and causes of death in the Marfan syndrome". The New England Journal of Medicine. 286 (15): 804–8. doi:10.1056/NEJM197204132861502. PMID 5011789. Retrieved 2010-12-22. Unknown parameter
|month=ignored (help) - ↑ 17.0 17.1 17.2 17.3 17.4 "Marfan Syndrome". Mayo Clinic. Retrieved January 12 2007. Unknown parameter
|dateformat=ignored (help); Check date values in:|accessdate=(help) - ↑ Braunwald's Heart Disease ~ A Textbook of Cardiovascular Medicine, Seventh Edition. United States of America: Elseview Saunders. 2005. p. 1894. ISBN 0-7216-0509-5. Unknown parameter
|coauthors=ignored (help) - ↑ "Marfan Syndrome, special concerns".
- ↑ Beighton P, de Paepe A, Danks D, Finidori G, Gedde-Dahl T, Goodman R, Hall JG, Hollister DW, Horton W, McKusick VA (1988). "International Nosology of Heritable Disorders of Connective Tissue, Berlin, 1986". American Journal of Medical Genetics. 29 (3): 581–94. doi:10.1002/ajmg.1320290316. PMID 3287925. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ De Paepe A, Devereux RB, Dietz HC, Hennekam RC, Pyeritz RE (1996). "Revised diagnostic criteria for the Marfan syndrome". American Journal of Medical Genetics. 62 (4): 417–26. doi:10.1002/(SICI)1096-8628(19960424)62:4<417::AID-AJMG15>3.0.CO;2-R. PMID 8723076. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Loeys BL, Dietz HC, Braverman AC, Callewaert BL, De Backer J, Devereux RB, Hilhorst-Hofstee Y, Jondeau G, Faivre L, Milewicz DM, Pyeritz RE, Sponseller PD, Wordsworth P, De Paepe AM (2010). "The revised Ghent nosology for the Marfan syndrome". Journal of Medical Genetics. 47 (7): 476–85. doi:10.1136/jmg.2009.072785. PMID 20591885. Retrieved 2010-12-22. Unknown parameter
|month=ignored (help) - ↑ Cocco G (2001). "Images in cardiology: The "thumb and wrist sign" in Marfan syndrome". Heart (British Cardiac Society). 86 (6): 602. PMC 1730018. PMID 11711445. Retrieved 2010-12-22. Unknown parameter
|month=ignored (help) - ↑ Freeman, Elaine (2007) "A Silver Bullet for Blake", Johns Hopkins Magazine, Fall, 2007.
- ↑ "Heart Surgery for Marfan Syndrome". Mayo Clinic.
- ↑ "Overview of the Nuss Procedure for Pectus Excavatum". Children's Hospital of The King's Daughters.
- ↑ "Dural Ectasia in the Marfan Spine: Symptoms and Treatment". Scoliosis Research Society.
- ↑ Habashi, Jennifer P. (April 7, 2006). "Losartan, an AT1 Antagonist, Prevents Aortic Aneurysm in a Mouse Model of Marfan Syndrome". 312 (5770): 117–121. doi:10.1126/science.1124287. Unknown parameter
|news=ignored (|newspaper=suggested) (help); Unknown parameter|abstract=ignored (help); Unknown parameter|coauthors=ignored (help) - ↑ "Atenolol vs. Losartan in Individuals with Marfan Syndrome Clinial Trial". National Marfan Foundation.
External links
- International Federation of Marfan Syndrome Organisations
- National Marfan Foundation (USA)
- Marfan diagnosis criteria
- National Institute for Health Marfan syndrome page (USA)
- Marfan Syndrome Center at medicinenet.com
- Marfan Syndrome Research - recent literature on Marfan Syndrome
- Marfan support
- Canadian Marfan Association
- Marfan Association UK
- Marfan de Mexico
- Norwegian Marfan Organization
- Marfan Life blog - mostly links to news articles about Marfan Syndrome
- MarfanSyndrome.Info - Findings in Marfan Syndrome and link collection
- Marfan-List - email discussion list for people and families with Marfan Syndrome
- South African Marfan Syndrome Organisation - support group for Africa
- Eye Findings in Marfan's syndrome
Template:Phakomatoses and other congenital malformations not elsewhere classified Template:SIB
ar:متلازمة مارفان de:Marfan-Syndrom ko:마르팡 증후군 it:Sindrome di Marfan he:תסמונת מרפן nl:Syndroom van Marfan nn:Marfans syndrom sr:Марфанов синдром fi:Marfanin oireyhtymä sv:Marfans syndrom uk:Синдром Марфана