Malathion: Difference between revisions
No edit summary |
No edit summary |
||
| Line 44: | Line 44: | ||
The presence or absence of ova at day 7 was not evaluated in these studies. The presence or absence of live lice or ova at 14 days following treatment was not evaluated in these studies. The residual amount of malathion on hair and scalp is unknown. | The presence or absence of ova at day 7 was not evaluated in these studies. The presence or absence of live lice or ova at 14 days following treatment was not evaluated in these studies. The residual amount of malathion on hair and scalp is unknown. | ||
|howSupplied=Malathion Lotion, USP 0.5%, is supplied in bottles of 2 fl. oz. (59 mL) | |howSupplied=Malathion Lotion, USP 0.5%, is supplied in bottles of 2 fl. oz. (59 mL) | ||
|storage=Store at controlled room temperature 20° to 25°C (68° to 77°F) | |storage=Store at controlled room temperature 20° to 25°C (68° to 77°F) | ||
|packLabel=[[file:File:Captura de pantalla 2014-12-29 a la(s) 12.00.46.png|thumb|none|600px]] | |packLabel=[[file:File:Captura de pantalla 2014-12-29 a la(s) 12.00.46.png|thumb|none|600px]] | ||
|alcohol=Alcohol-Malathion interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Malathion interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
|brandNames=*[[Ovide]] | |||
}} | |||
{{PillImage | |||
|fileName=Captura de pantalla 2014-12-29 a la(s) 12.03.41.png | |||
}} | }} | ||
{{Chembox new | {{Chembox new | ||
Revision as of 17:04, 29 December 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Malathion is an organophosphate that is FDA approved for the treatment of pediculus humanus capitis (Pediculus humanus capitis). Common adverse reactions include application site irritation, skin irritation.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- (1)Apply Malathion Lotion on dry hair in amount just sufficient to thoroughly wet the hair and scalp. Pay particular attention to the back of the head and neck while applying Malathion lotion. Wash hands after applying to scalp.
- (2)Allow hair to dry naturally - use no electric heat source, and allow hair to remain uncovered.
- (3)After 8 to 12 hours, the hair should be shampooed.
- (4)Rinse and use a fine - toothed (nit) comb to remove dead lice and eggs.
- (5)If lice are still present after 7 - 9 days, repeat with a second application of Malathion Lotion.
- (6)Further treatment is generally not necessary. Other family members should be evaluated by a physician to determine if infested, and if so, receive treatment.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Infestation by Phthirus pubis
- Dosage: Malathion 0.5% lotion applied for 8 to 12 hours and washed off[1]
Non–Guideline-Supported Use
Infestation by Phthirus pubis
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Malathion FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Malathion in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Malathion in pediatric patients.
Contraindications
There is limited information regarding Malathion Contraindications in the drug label.
Warnings
There is limited information regarding Malathion Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
Dermatologic Effects
- Irritation of Skin and Scalp
Ophthalmologic Effects
Postmarketing Experience
There is limited information regarding Malathion Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Malathion Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): B There was no evidence of teratogenicity in studies in rats and rabbits at doses up to 900 mg/kg/day and 100 mg/kg/day malathion, respectively. A study in rats failed to show any gross fetal abnormalities attributable to feeding malathion up to 2,500 ppm (∼ 200 mg/kg/day) in the diet during a three-generation evaluation period.
These doses were approximately 40 to 180 times higher than the dose anticipated in a 60 kg adult (based on body surface area and assuming 100% bioavailability). Because animal reproduction studies are not always predictive of human responses, this drug should be used (or handled) during pregnancy only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Malathion in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Malathion during labor and delivery.
Nursing Mothers
Malathion in an acetone vehicle has been reported to be absorbed through human skin to the extent of 8% of the applied dose. However, percutaneous absorption from the Malathion Lotion, 0.5% formulation has not been studied, and it is not known whether malathion is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Malathion Lotion is administered to (or handled by) a nursing mother.
Pediatric Use
The safety and effectiveness of Malathion Lotion in children less than 6 years of age has not been established via well-controlled trials.
Geriatic Use
There is no FDA guidance on the use of Malathion in geriatric settings.
Gender
There is no FDA guidance on the use of Malathion with respect to specific gender populations.
Race
There is no FDA guidance on the use of Malathion with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Malathion in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Malathion in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Malathion in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Malathion in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Malathion Administration in the drug label.
Monitoring
There is limited information regarding Malathion Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Malathion and IV administrations.
Overdosage
Consideration should be given, as part of the treatment program, to the high concentration of isopropyl alcohol in the vehicle. Malathion, although a weaker cholinesterase inhibitor than some other organophosphates, may be expected to exhibit the same symptoms of cholinesterase depletion after accidental ingestion orally. If accidentally swallowed, vomiting should be induced promptly or the stomach lavaged with 5% sodium bicarbonate solution. Severe respiratory distress is the major and most serious symptom of organophosphate poisoning requiring artificial respiration, and atropine may be needed to counteract the symptoms of cholinesterase depletion.
Repeat analyses of serum and RBC cholinesterase may assist in establishing the diagnosis and formulating a long-range prognosis.
Pharmacology
Mechanism of Action
Malathion is an organophosphate agent which acts as a pediculicide by inhibiting cholinesterase activity in vivo.
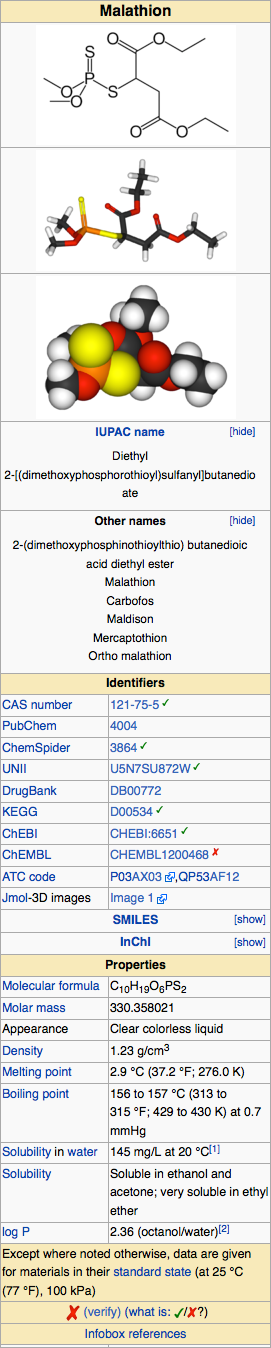
Structure
The chemical name of malathion is (±) - [(dimethoxyphosphinothioyl) - thio] butanedioic acid diethyl ester. Malathion has a molecular weight of 330.36, represented by C10H19O6PS2, and has the following chemical structure:
Pharmacodynamics
There is limited information regarding Malathion Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Malathion Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Malathion Nonclinical Toxicology in the drug label.
Clinical Studies
Two controlled clinical trials evaluated the pediculicidal activity of Malathion Lotion. Patients applied the lotion to the hair and scalp in quantities, up to a maximum of 2 fl. oz., sufficient to thoroughly wet the hair and scalp. The lotion was allowed to air dry and was shampooed with Prell shampoo 8 to 12 hours after application. Patients in both the Malathion Lotion group and in the vehicle group were examined immediately after shampooing, 24 hours after, and 7 days after for the presence of live lice. Results are shown in the following table:
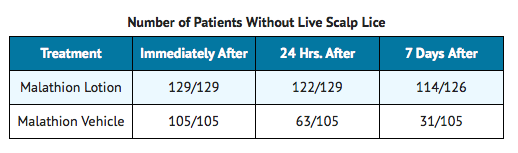
The presence or absence of ova at day 7 was not evaluated in these studies. The presence or absence of live lice or ova at 14 days following treatment was not evaluated in these studies. The residual amount of malathion on hair and scalp is unknown.
How Supplied
Malathion Lotion, USP 0.5%, is supplied in bottles of 2 fl. oz. (59 mL)
Storage
Store at controlled room temperature 20° to 25°C (68° to 77°F)
Images
Drug Images
{{#ask: Page Name::Malathion |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Malathion |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Malathion Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Malathion interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Malathion Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Malathion
|Pill Name=Captura de pantalla 2014-12-29 a la(s) 12.03.41.png
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
Malathion is an organophosphate parasympathomimetic which binds irreversibly to cholinesterase. Malathion is an insecticide of relatively low human toxicity.
In the former USSR it was known as carbophos, in New Zealand and Australia as maldison and in South Africa as mercaptothion. [1]
Risks
Malathion breaks down into malaoxon, which is 60 times more toxic than malathion. For this reason, if malathion is used in or somehow enters an indoor environment, as it breaks down into malaoxon, it can seriously and chronically poison the occupants living or working in this environment. Malathion present in untreated water is converted to malaoxon during the chlorination phase of water treatment, so malathion should not be used in waters that may be used as a source for drinking water, or any upstream waters.
In 1976, numerous malaria workers in Pakistan were poisoned by isomalathion, a common impurity in malathion,[2] which is capable of inhibiting carboxyesterase enzymes in those exposed to it; the original toxicity evaluation for malathion had not anticipated isomalathion coexposure.
Clinical uses
Malathion is used as a treatment for head lice, body lice and scabies. It effectively kills both the eggs and the adult lice.
Instances of use
In the US, it is the most commonly used organophosphate insecticide. [3]
Malathion was used in the 1980s in California to combat the Mediterranean Fruit Fly. This was accomplished on a wide scale by the near weekly aerial spraying of suburban communities for a period of several months. Formations of three or four agricultural helicopters would overfly suburban portions of San Bernardino county releasing a mixture of malathion and corn syrup, the corn syrup being a bait for the fruit flies. Malathion has also been used to combat the Mediterranean Fruit Fly in Australia.[4]
Malathion was sprayed in many cities to combat West Nile virus. In the Fall of 1999 and the Spring of 2000, Long Island and the five boroughs of New York City were sprayed with malathion. Use of the insecticide has been blamed for large lobster die-off in Long Island Sound.[5]
Manitoba, Province of Canada, ordered the city of Winnipeg, Manitoba to be sprayed in July 2005 as part of the West Nile virus campaign. Prior to this, Malathion was used over the last couple of decades on regular basis during summer months to kill nuisance mosquitos, but homeowners were allowed to exempt their properties if they chose. Today, Winnipeg is the only major city in Canada with an ongoing Malathion nuisance adult mosquito control program.
Malathion is also used in conjunction with diesel to fog an area where there are plenty of mosquitoes. By diluting the mixture, it becomes much weaker. It is possible to dilute the mixture to the point where mosquitoes are not killed, but become more resistant to the mixture, making it less effective in subsequent foggings.
See also
References
- ↑ "alanwood.net". Retrieved 2007-09-16.
- ↑ Baker EL, Warren M, Zack M; et al. (1978). "Epidemic malathion poisoning in Pakistan malaria workers". Lancet. 1 (8054): 31–4. PMID 74508.
- ↑ Bonner MR, Coble J, Blair A; et al. (2007). "Malathion Exposure and the Incidence of Cancer in the Agricultural Health Study". doi:10.1093/aje/kwm182. PMID 17720683.
- ↑ Edwards JW, Lee SG, Heath LM, Pisaniello DL (2007). "Worker exposure and a risk assessment of malathion and fenthion used in the control of Mediterranean fruit fly in South Australia". Environ. Res. 103 (1): 38–45. doi:10.1016/j.envres.2006.06.001. PMID 16914134.
- ↑ http://www.seagrant.sunysb.edu/LILobsters/Oct04Meeting/summaries/LISLISummary-Pesticides.pdf
cs:Malation de:Malathion fa:مالاتیون nl:Malathion