Macitentan
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gerald Chi
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: EMBRYO-FETAL TOXICITY
See full prescribing information for complete Boxed Warning.
* Do not administer Opsumit to a pregnant female because it may cause fetal harm.
|
Overview
Macitentan is an endothelin receptor antagonist and antihypertensive that is FDA approved for the {{{indicationType}}} of pulmonary artery hypertension. There is a Black Box Warning for this drug as shown here. Common adverse reactions include anemia, influenza, headache, urinary tract infections, bronchitis, nasopharyngitis, and pharyngitis.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Pulmonary Arterial Hypertension
- Opsumit® is an endothelin receptor antagonist (ERA) indicated for the treatment of pulmonary arterial hypertension (PAH, WHO Group I) to delay disease progression. Disease progression included: death, initiation of intravenous (IV) or subcutaneous prostanoids, or clinical worsening of PAH (decreased 6-minute walk distance, worsened PAH symptoms and need for additional PAH treatment). Opsumit also reduced hospitalization for PAH.
- Effectiveness was established in a long-term study in PAH patients with predominantly WHO Functional Class II-III symptoms treated for an average of 2 years. Patients were treated with Opsumit monotherapy or in combination with phosphodiesterase-5 inhibitors or inhaled prostanoids. Patients had idiopathic and heritable PAH (57%), PAH caused by connective tissue disorders (31%), and PAH caused by congenital heart disease with repaired shunts (8%).
- Dosing Information
- The recommended dosage of Opsumit is 10 mg once daily for oral administration. Doses higher than 10 mg once daily have not been studied in patients with PAH and are not recommended.
- Initiate treatment with Opsumit in females of reproductive potential only after a negative pregnancy test. Obtain monthly pregnancy test during treatment.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Macitentan in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Macitentan in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Macitentan in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- The safety and efficacy of Opsumit in children have not been established.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Macitentan in pediatric patients.
Contraindications
- Pregnancy
- Opsumit may cause fetal harm when administered to a pregnant woman. Opsumit is contraindicated in females who are pregnant. Opsumit was consistently shown to have teratogenic effects when administered to animals. If Opsumit is used during pregnancy, apprise the patient of the potential hazard to a fetus.
Warnings
|
WARNING: EMBRYO-FETAL TOXICITY
See full prescribing information for complete Boxed Warning.
* Do not administer Opsumit to a pregnant female because it may cause fetal harm.
|
- Embryo-fetal Toxicity
- Opsumit may cause fetal harm when administered during pregnancy and is contraindicated for use in females who are pregnant. In females of reproductive potential, exclude pregnancy prior to initiation of therapy, ensure use of acceptable contraceptive methods and obtain monthly pregnancy tests.
- Opsumit is available for females through the Opsumit REMS Program, a restricted distribution program.
- Opsumit REMS Program
- For all females, Opsumit is available only through a restricted program called the Opsumit REMS Program, because of the risk of embryo-fetal toxicity.
- Notable requirements of the Opsumit REMS Program include the following:
- Prescribers must be certified with the program by enrolling and completing training.
- All females, regardless of reproductive potential, must enroll in the Opsumit REMS Program prior to initiating Opsumit. Male patients are not enrolled in the REMS.
- Females of reproductive potential must comply with the pregnancy testing and contraception requirements.
- Pharmacies must be certified with the program and must only dispense to patients who are authorized to receive Opsumit.
- Further information is available at www.OpsumitREMS.com or 1-866-228-3546. Information on Opsumit certified pharmacies or wholesale distributors is available through Actelion Pathways at 1-866-228-3546.
- Hepatotoxicity
- Other ERAs have caused elevations of aminotransferases, hepatotoxicity, and liver failure. The incidence of elevated aminotransferases in the study of Opsumit in PAH is shown in Table 1.
- In the placebo-controlled study of Opsumit, discontinuations for hepatic adverse events were 3.3% in the Opsumit 10 mg group vs. 1.6% for placebo. Obtain liver enzyme tests prior to initiation of Opsumit and repeat during treatment as clinically indicated.
- Advise patients to report symptoms suggesting hepatic injury (nausea, vomiting, right upper quadrant pain, fatigue, anorexia, jaundice, dark urine, fever, or itching). If clinically relevant aminotransferase elevations occur, or if elevations are accompanied by an increase in bilirubin >2 × ULN, or by clinical symptoms of hepatotoxicity, discontinue Opsumit. Consider re-initiation of Opsumit when hepatic enzyme levels normalize in patients who have not experienced clinical symptoms of hepatotoxicity.
- Hemoglobin Decrease
- Decreases in hemoglobin concentration and hematocrit have occurred following administration of other ERAs and were observed in clinical studies with Opsumit. These decreases occurred early and stabilized thereafter. In the placebo-controlled study of Opsumit in PAH, Opsumit 10 mg caused a mean decrease in hemoglobin from baseline to up to 18 months of about 1.0 g/dL compared to no change in the placebo group. A decrease in hemoglobin to below 10.0 g/dL was reported in 8.7% of the Opsumit 10 mg group and in 3.4% of the placebo group. Decreases in hemoglobin seldom require transfusion. Initiation of Opsumit is not recommended in patients with severe anemia. Measure hemoglobin prior to initiation of treatment and repeat during treatment as clinically indicated.
- Pulmonary Edema with Pulmonary Veno-occlusive Disease (PVOD)
- Should signs of pulmonary edema occur, consider the possibility of associated PVOD. If confirmed, discontinue Opsumit.
- Decreased Sperm Counts
- Other ERAs have caused adverse effects on spermatogenesis. Counsel men about potential effects on fertility.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
- Safety data for Opsumit were obtained primarily from one placebo-controlled clinical study in 742 patients with PAH (SERAPHIN study). The exposure to Opsumit in this trial was up to 3.6 years with a median exposure of about 2 years (N=542 for 1 year; N=429 for 2 years; and N=98 for more than 3 years). The overall incidence of treatment discontinuations because of adverse events was similar across Opsumit 10 mg and placebo treatment groups (approximately 11%).
- Table 2 presents adverse reactions more frequent on Opsumit than on placebo by ≥3%.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Macitentan in the drug label.
Drug Interactions
- Strong CYP3A4 Inducers
- Strong CYP3A4 Inhibitors
- Concomitant use of strong CYP3A4 inhibitors like ketoconazole approximately double macitentan exposure. Many HIV drugs like ritonavir are strong inhibitors of CYP3A4. Avoid concomitant use of Opsumit with strong CYP3A4 inhibitors. Use other PAH treatment options when strong CYP3A4 inhibitors are needed as part of HIV treatment.
In vitro studies
- At plasma levels obtained with dosing at 10 mg once daily, macitentan has no relevant inhibitory or inducing effects on CYP enzymes, and is neither a substrate nor an inhibitor of the multi-drug resistance protein (P-gp, MDR-1). Macitentan and its active metabolite are neither substrates nor inhibitors of the organic anion transporting polypeptides (OATP1B1 and OATP1B3) and do not significantly interact with proteins involved in hepatic bile salt transport, i.e., the bile salt export pump (BSEP) and the sodium-dependent taurocholate co-transporting polypeptide (NTCP).
In vivo studies
- Effect of other drugs on macitentan: The effect of other drugs on macitentan and its active metabolite are studied in healthy subjects and are shown in Figure 1 below.
- Effects of other strong CYP3A4 inhibitors such as ritonavir on macitentan were not studied, but are likely to result in an increase in macitentan exposure at steady state similar to that seen with ketoconazole [see Drug Interactions (7.2)].
Effect of macitentan on other drugs
- Warfarin: Macitentan once daily dosing did not alter the exposure to R- and S-warfarin or their effect on international normalized ratio (INR).
- Sildenafil: At steady-state, the exposure to sildenafil 20 mg t.i.d. increased by 15% during concomitant administration of macitentan 10 mg once daily. This change is not considered clinically relevant.
Use in Specific Populations
Pregnancy
- Pregnancy Category X
- Risk Summary
- Opsumit may cause fetal harm when administered to a pregnant woman and is contraindicated during pregnancy. Macitentan was teratogenic in rabbits and rats at all doses tested. A no-effect dose was not established in either species. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, advise the patient of the potential hazard to a fetus.
- Animal Data
- In both rabbits and rats, there were cardiovascular and mandibular arch fusion abnormalities. Administration of macitentan to female rats from late pregnancy through lactation caused reduced pup survival and impairment of the male fertility of the offspring at all dose levels tested.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Macitentan in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Macitentan during labor and delivery.
Nursing Mothers
- It is not known whether Opsumit is present in human milk. Macitentan and its metabolites were present in the milk of lactating rats. Because many drugs are present in human milk and because of the potential for serious adverse reactions from macitentan in nursing infants, nursing mothers should discontinue nursing or discontinue Opsumit.
Pediatric Use
- The safety and efficacy of Opsumit in children have not been established.
Geriatic Use
- Of the total number of subjects in the clinical study of Opsumit for PAH, 14% were 65 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects.
Gender
There is no FDA guidance on the use of Macitentan with respect to specific gender populations.
Race
There is no FDA guidance on the use of Macitentan with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Macitentan in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Macitentan in patients with hepatic impairment.
Females of Reproductive Potential and Males
- Females
- Pregnancy Testing: Female patients of reproductive potential must have a negative pregnancy test prior to starting treatment with Opsumit and monthly pregnancy tests during treatment with Opsumit. Advise patients to contact their health care provider if they become pregnant or suspect they may be pregnant. Perform a pregnancy test if pregnancy is suspected for any reason. For positive pregnancy tests, counsel patients on the potential risk to the fetus.
- Contraception: Female patients of reproductive potential must use acceptable methods of contraception during treatment with Opsumit and for 1 month after treatment with Opsumit. Patients may choose one highly effective form of contraception (intrauterine devices (IUD), contraceptive implants or tubal sterilization) or a combination of methods (hormone method with a barrier method or two barrier methods). If a partner's vasectomy is the chosen method of contraception, a hormone or barrier method must be used along with this method. Counsel patients on pregnancy planning and prevention, including emergency contraception, or designate counseling by another healthcare provider trained in contraceptive counseling.
- Males
- Testicular effects: Like other endothelin receptor antagonists, Opsumit may have an adverse effect on spermatogenesis
Immunocompromised Patients
There is no FDA guidance one the use of Macitentan in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Macitentan in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Macitentan in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Opsumit has been administered as a single dose of up to and including 600 mg to healthy subjects (60 times the approved dosage). Adverse reactions of headache, nausea and vomiting were observed.
Management
- In the event of an overdose, standard supportive measures should be taken, as required. Dialysis is unlikely to be effective because macitentan is highly protein-bound.
Chronic Overdose
There is limited information regarding Chronic Overdose of Macitentan in the drug label.
Pharmacology
| |
Macitentan
| |
| Systematic (IUPAC) name | |
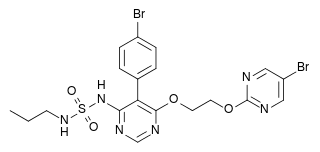
| N-[5-(4-Bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl]-N'-propylsulfamide | |
| Identifiers | |
| CAS number | |
| ATC code | C02 |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 588.273 g/mol |
| SMILES | & |
| Synonyms | ACT-064992 |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | Hydrolysis, oxidation (CYP3A4) |
| Half life | ? |
| Excretion | 2/3 urine, 1/3 faeces |
| Therapeutic considerations | |
| Pregnancy cat. |
X(US) |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) FDA approved drug |
| Routes | Oral |
Mechanism of Action
- Endothelin (ET)-1 and its receptors (ETA and ETB) mediate a variety of deleterious effects, such as vasoconstriction, fibrosis, proliferation, hypertrophy, and inflammation. In disease conditions such as PAH, the local ET system is upregulated and is involved in vascular hypertrophy and in organ damage.
- Macitentan is an endothelin receptor antagonist that prevents the binding of ET-1 to both ETA and ETB receptors. Macitentan displays high affinity and sustained occupancy of the ET receptors in human pulmonary arterial smooth muscle cells. One of the metabolites of macitentan is also pharmacologically active at the ET receptors and is estimated to be about 20% as potent as the parent drug in vitro.
Structure
- Opsumit (macitentan) is an endothelin receptor antagonist. The chemical name of macitentan is N-[5-(4-Bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl]-N'-propylsulfamide. It has a molecular formula of C19H20Br2N6O4S and a molecular weight of 588.27. Macitentan is achiral and has the following structural formula:
- Macitentan is a crystalline powder that is insoluble in water. In the solid state macitentan is very stable, is not hygroscopic, and is not light sensitive.
- Opsumit is available as a 10 mg film-coated tablet for once daily oral administration. The tablets include the following inactive ingredients: lactose monohydrate, magnesium stearate, microcrystalline cellulose, polysorbate 80, povidone, and sodium starch glycolate Type A. The tablets are film-coated with a coating material containing polyvinyl alcohol, soya lecithin, talc, titanium dioxide, and xanthan gum.
Pharmacodynamics
- Pulmonary Hemodynamics: The clinical efficacy study in patients with pulmonary arterial hypertension assessed hemodynamic parameters in a subset of patients after 6 months of treatment. Patients treated with Opsumit 10 mg (N=57) achieved a median reduction of 37% (95% CI 22-49) in pulmonary vascular resistance and an increase of 0.6 L/min/m2 (95% CI 0.3-0.9) in cardiac index compared to placebo (N=67).
- Cardiac Electrophysiology: In a randomized, placebo-controlled four-way crossover study with a positive control in healthy subjects, repeated doses of macitentan 10 and 30 mg (3 times the recommended dosage) had no significant effect on the QTc interval.
Pharmacokinetics
- The pharmacokinetics of macitentan and its active metabolite have been studied primarily in healthy subjects. The pharmacokinetics of macitentan are dose proportional over a range from 1 mg to 30 mg after once daily administration.
- A cross study comparison shows that the exposures to macitentan and its active metabolite in patients with PAH are similar to those observed in healthy subjects.
Absorption and Distribution
- The maximum plasma concentration of macitentan is achieved about 8 hours after oral administration. The absolute bioavailability after oral administration is not known. In a study in healthy subjects, the exposure to macitentan and its active metabolite were unchanged after a high fat breakfast. Macitentan may therefore be taken with or without food.
- Macitentan and its active metabolite are highly bound to plasma proteins (>99%), primarily to albumin and to a lesser extent to alpha-1-acid glycoprotein. The apparent volumes of distribution (Vss/F) of macitentan and its active metabolite were about 50 L and 40 L respectively in healthy subjects.
Metabolism and Elimination
- Following oral administration, the apparent elimination half-lives of macitentan and its active metabolite are approximately 16 hours and 48 hours, respectively. Macitentan is metabolized primarily by oxidative depropylation of the sulfamide to form the pharmacologically active metabolite. This reaction is dependent on the cytochrome P450 (CYP) system, mainly CYP3A4 with a minor contribution of CYP2C19. At steady state in PAH patients, the systemic exposure to the active metabolite is 3-times the exposure to macitentan and is expected to contribute approximately 40% of the total pharmacologic activity. In a study in healthy subjects with radiolabeled macitentan, approximately 50% of radioactive drug material was eliminated in urine but none was in the form of unchanged drug or the active metabolite. About 24% of the radioactive drug material was recovered from feces.
Special Populations
- There are no clinically relevant effects of age, sex, or race on the pharmacokinetics of macitentan and its active metabolite.
- Renal impairment: Exposure to macitentan and its active metabolite in patients with severe renal impairment (CrCl 15-29 mL/min) compared to healthy subjects was increased by 30% and 60%, respectively. This increase is not considered clinically relevant.
- Hepatic impairment: Exposure to macitentan was decreased by 21%, 34%, and 6% and exposure to the active metabolite was decreased by 20%, 25%, and 25% in subjects with mild, moderate, or severe hepatic impairment (Child-Pugh Class A, B, and C), respectively. This decrease is not considered clinically relevant.
Nonclinical Toxicology
- Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenesis: Carcinogenicity studies of 2 years' duration did not reveal any carcinogenic potential at exposures 75-fold and 140-fold the human exposure (based on AUC) in male and female mice, respectively, and 8.3- and 42-fold in male and female rats, respectively.
- Mutagenesis: Macitentan was not genotoxic in a standard battery of in vitro and in vivo assays that included a bacterial reverse mutation assay, an assay for gene mutations in mouse lymphoma cells, a chromosome aberration test in human lymphocytes, and an in vivo micronucleus test in rats.
- Impairment of Fertility: Treatment of juvenile rats from postnatal Day 4 to Day 114 led to reduced body weight gain and testicular tubular atrophy at exposures 7-fold the human exposure. Fertility was not affected.
- Reversible testicular tubular dilatation was observed in chronic toxicity studies at exposures greater than 7-fold and 23-fold the human exposure in rats and dogs, respectively. After 2 years of treatment, tubular atrophy was seen in rats at 4-fold the human exposure. Macitentan did not affect male or female fertility at exposures ranging from 19- to 44-fold the human exposure, respectively, and had no effect on sperm count, motility, and morphology in male rats. No testicular findings were noted in mice after treatment up to 2 years.
- Animal Toxicology
- In dogs, macitentan decreased blood pressure at exposures similar to the therapeutic human exposure. Intimal thickening of coronary arteries was observed at 17-fold the human exposure after 4 to 39 weeks of treatment. Due to the species-specific sensitivity and the safety margin, this finding is considered not relevant for humans.
- There were no adverse liver findings in long-term studies conducted in mice, rats, and dogs at exposures of 12- to 116-fold the human exposure.
Clinical Studies
- Pulmonary Arterial Hypertension
- The effect of macitentan on progression of PAH was demonstrated in a multi-center, long-term (average duration of exposure approximately 2 years), placebo-controlled study in 742 patients with symptomatic [WHO functional class (FC) II-IV] PAH who were randomized to placebo (n=250), 3 mg macitentan (n=250), or 10 mg macitentan (n=242) once daily.
- The primary study endpoint was time to the first occurrence of death, a significant morbidity event, defined as atrial septostomy, lung transplantation, initiation of IV or subcutaneous (SC) prostanoids, or "other worsening of PAH" during double-blind treatment plus 7 days. Other worsening was defined as all of the following: 1) a sustained ≥15% decrease from baseline in 6 minute walk distance (6MWD), 2) worsening of PAH symptoms (worsening of WHO FC), and 3) need for additional treatment for PAH. All of these other worsening events were confirmed by an independent adjudication committee, blinded to treatment allocation. A critical secondary endpoint was time to PAH death or PAH hospitalization.
- The mean patient age was 46 years (14% were age 65 or above). Most patients were white (55%) or Asian (29%) and female (77%). Approximately 52%, 46%, and 2% of patients were in WHO FC II, III, and IV, respectively.
- Idiopathic or heritable PAH was the most common etiology in the study population (57%) followed by PAH caused by connective tissue disorders (31%), PAH caused by congenital heart disease with repaired shunts (8%), and PAH caused by other etiologies [drugs and toxins (3%) and HIV (1%)].
- At baseline, the majority of enrolled patients (64%) were being treated with a stable dose of specific therapy for PAH, either oral phosphodiesterase inhibitors (61%) and/or inhaled/oral prostanoids (6%).
- Study results are described for the placebo and Opsumit 10 mg groups. The median treatment durations were 101 and 118 weeks in the placebo and Opsumit 10 mg groups, respectively, up to a maximum of 188 weeks.
- Treatment with Opsumit 10 mg resulted in a 45% reduction (HR 0.55, 97.5% CI 0.39-0.76; logrank p<0.0001) in the occurrence of the primary endpoint up to end of double-blind treatment compared to placebo (Table 3 and Figure 2). The beneficial effect of Opsumit 10 mg was primarily attributable to a reduction in clinical worsening events (deterioration in 6MWD and worsening of PAH symptoms and need for additional PAH treatment).
- Subgroup analyses were performed to examine their influence on outcome as shown in Figure 3. Consistent efficacy of Opsumit 10 mg on the primary endpoint was seen across subgroups of age, sex, race, etiology, by monotherapy or in combination with another PAH therapy, baseline 6MWD, and baseline WHO FC.
- Treatment with Opsumit 10 mg resulted in a placebo-corrected mean increase in 6MWD of 22 meters at Month 6 (97.5% CI 3-41; p=0.0078), with significant improvement in 6MWD by Month 3. 6MWD increased more in patients with worse baseline WHO Functional Class (37 meters and 12 meters placebo-corrected mean increase in WHO FC III/IV and FC I/II, respectively). The increase in 6MWD achieved with Opsumit was maintained for the duration of the study.
- Treatment with Opsumit 10 mg led to an improvement of at least one WHO Functional Class at Month 6 in 22% of patients compared to 13% of patients treated with placebo.
How Supplied
- Opsumit tablets are 10 mg white, film-coated, bi-convex debossed with "10" on one side and supplied as follows:
- 15 count /PVC/ PE/PVDC aluminum foil blisters in carton (NDC 66215-501-15)
- 30 count white high-density polyethylene bottle in carton (NDC 66215-501-30)
- Store at 20°C to 25°C (68°F to 77°F). Excursions are permitted between 15°C and 30°C (59°F and 86°F).
Storage
There is limited information regarding Macitentan Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Macitentan |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Macitentan |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information

Precautions with Alcohol
- Alcohol-Macitentan interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Opsumit®[1]
Look-Alike Drug Names
- N/A[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "Opsumit (macitentan) tablet, film coated".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Macitentan |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Macitentan |Label Name=Macitentan10.png
}}
{{#subobject:
|Label Page=Macitentan |Label Name=Macitentan11.png
}}







