Lymphoplasmacytic lymphoma medical therapy: Difference between revisions
Sara Mohsin (talk | contribs) |
|||
| (29 intermediate revisions by 3 users not shown) | |||
| Line 4: | Line 4: | ||
==Overview== | ==Overview== | ||
[[Risk stratification tools|Risk stratification]] determines the protocol of management used for [[lymphoplasmacytic lymphoma]]. There is no treatment for [[asymptomatic]] [[lymphoplasmacytic lymphoma]]. The mainstay of treatment for [[symptomatic]] [[lymphoplasmacytic lymphoma]] is [[Rituximab]] +/- [[Chemotherapy]]. [[Hyperviscosity syndrome]] is a [[medical emergency]] and requires prompt treatment with [[plasmapheresis]]. | [[Risk stratification tools|Risk stratification]] determines the [[Protocol (natural sciences)|protocol]] of management used for [[lymphoplasmacytic lymphoma]]. There is no [[Treatments|treatment]] for [[asymptomatic]] [[lymphoplasmacytic lymphoma]]. The mainstay of [[Treatments|treatment]] for [[symptomatic]] [[lymphoplasmacytic lymphoma]] is [[Rituximab]] +/- [[Chemotherapy]]. [[Hyperviscosity syndrome]] is a [[medical emergency]] and requires [[prompt]] [[Treatments|treatment]] with [[plasmapheresis]]. [[Drug]] of choice for the [[Treatments|treatment]] of [[Bing-Neel syndrome|bing-neel syndrome]] is [[Ibrutinib]] with or without concurrent [[rituximab]]. Other [[Treatments|treatment]] options include [[targeted therapy]], [[immunotherapy]] and [[radiation therapy]]. | ||
==Medical Therapy== | ==Medical Therapy== | ||
There's no [[cure]] for | There's no [[cure]] for LPL with [[current]] [[Therapy|therapies]]. Instead, the [[Treatments|treatment]] [[Goal-directed therapy|goals]] are to [[control]] [[symptoms]] and [[Prevention (medical)|prevent]] [[End organ damage|end-organ damage]], while [[Maximum|maximizing]] [[quality of life]]. There is no [[standard]] [[therapy]] for the [[Treatments|treatment]] of LPL. While various [[drugs]] and [[Combination therapy|combinations]] have demonstrated to have provided [[clinical]] benefit, hence, there are several different options for [[Treatments|treating]] [[lymphoplasmacytic lymphoma]] [[Dependent variable|depending]] on [[Staging (pathology)|stage]] of the [[disease]]:<ref name="Tx">Lymphoplasmacytic lymphoma. Canadian Cancer Society 2015. http://www.cancer.ca/en/cancer-information/cancer-type/non-hodgkin-lymphoma/non-hodgkin-lymphoma/types-of-nhl/lymphoplasmacytic-lymphoma/?region=ab Accessed on November 6 2015 </ref> | ||
{| class="wikitable" | |||
|+Summary of how to approach different patients with lymphoplasmacytic lymphoma | |||
! style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|Patient's condition/parameters}} | |||
! style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|How to proceed accordingly}} | |||
|- | |||
| | |||
* [[Immunoglobulin M|IgM]] [[MGUS]] (<10% lymphoplasmacytic [[Infiltration (medical)|infiltrate]]) | |||
* Smoldering/[[asymptomatic]] [[lymphoplasmacytic lymphoma]] | |||
* [[Hemoglobin]] < or = 11g/dl | |||
* [[Platelets]] > or = 120x10*9 /L | |||
|[[Observation]] | |||
|- | |||
| | |||
* [[Symptomatic]] LPL [[patient]] | |||
* [[Hemoglobin]] <11g/dl | |||
* [[Platelets]] <120x10*9 /L | |||
* [[IgM]]-related [[neuropathy]] | |||
* [[Hemolytic anemia]] [[Association (statistics)|associated]] with [[Waldenström's macroglobulinemia|Waldenstrom macroglobulinemia]] | |||
| | |||
*[[Treatments|Treat]] with single [[Agent study|agent]], [[rituximab]] (one [[Cycle (gene)|cycle]] only, no [[Maintenance dose|maintenance]] [[therapy]] required) | |||
* [[Plasmapheresis]] in [[Case-based reasoning|case]] of occurrence of [[hyperviscosity]] with [[Treatments|treatment]] | |||
|- | |||
| | |||
*[[Bulk density|Bulky]] [[disease]] | |||
* [[Hemoglobin]] < or = 10g/dl | |||
* [[Platelets]] < 100x10*9/L | |||
* Constitutional [[symptoms]] | |||
* [[Hyperviscosity syndrome]] | |||
|Hperviscosity present: | |||
* [[Plasmapheresis]] and DRC ([[dexamethasone]] + [[rituximab]] + [[cyclophosphamide]]) | |||
[[Hyperviscosity]] absent: | |||
* DRC only | |||
|- | |||
| | |||
* [[Patient]] with [[relapse]] of [[lymphoplasmacytic lymphoma]] (mSMART [[Guideline (medical)|guidelines]]) | |||
|Consider [[clinical trial]] + [[stem cell transplant]] in selected [[patients]]: | |||
* If the [[length]] of [[Response element|response]] to initial [[therapy]] is = or >2 [[Year|years]], [[Repeatability|repeat]] the same [[First-line therapy|first-line]] [[Agent study|agent]] as used before | |||
* If the [[length]] of [[Response element|response]] to initial [[therapy]] is <2 years, use an [[Alternative medicine|alternative]] [[First-line therapy|first-line]] [[Agent study|agent]] | |||
|} | |||
====Watchful waiting/active surveillance for asymptomatic patients with LPL | ====Watchful waiting/active surveillance for asymptomatic patients with LPL==== | ||
There is no treatment for [[asymptomatic]] patients with LPL. As LPL develops slowly and may not need to be treated right away, it is monitored by [[Health care|healthcare]] team every 3-6 months which is known as [[watchful waiting]]/active surveillance and treatment is started when [[symptoms]] appear, such as [[hyperviscosity syndrome]], or there are [[signs]] that the [[disease]] is progressing more quickly.<ref name="BM">Waldenström's macroglobulinemia. Patient (2015)http://patient.info/doctor/waldenstroms-macroglobulinaemia-pro Accessed on November 10, 2015</ref> Active surveillance includes monitoring of the following laboratory parameters: | There is no [[Treatments|treatment]] for [[asymptomatic]] [[patients]] with LPL. As LPL [[Development|develops]] [[Slow|slowly]] and may not need to be [[Treatments|treated]] right away, it is [[Monitor unit|monitored]] by [[Health care|healthcare]] team every 3-6 months which is known as [[watchful waiting]]/active surveillance and [[Treatments|treatment]] is started when [[symptoms]] [[Appearance|appear]], such as [[hyperviscosity syndrome]], or there are [[signs]] that the [[disease]] is progressing more quickly.<ref name="BM">Waldenström's macroglobulinemia. Patient (2015)http://patient.info/doctor/waldenstroms-macroglobulinaemia-pro Accessed on November 10, 2015</ref> Active surveillance includes [[Monitoring competence|monitoring]] of the following [[laboratory]] [[Parameter|parameters]]: | ||
*[[Complete blood count]] ([[Complete blood count|CBC]]) with differential | *[[Complete blood count]] ([[Complete blood count|CBC]]) with [[Difference (philosophy)|differential]] | ||
*Complete metabolic panel ([[CMP-N-acetylneuraminate monooxygenase|CMP]]) | *Complete [[metabolic]] [[Panel analysis|panel]] ([[CMP-N-acetylneuraminate monooxygenase|CMP]]) | ||
*[[Immunoglobulin]] levels in the [[serum]] (quantitative) | *[[Immunoglobulin]] levels in the [[serum]] ([[quantitative]]) | ||
*[[Serum protein electrophoresis]] | *[[Serum protein electrophoresis]] | ||
====Symptomatic patients with LPL | ====Symptomatic patients with LPL==== | ||
[[Symptomatic]] patients with LPL are started on [[chemotherapy]] depending on the stage.<ref name="ADR">Waldenström's macroglobulinemia: prognosis and management. Blood Cancer Journal (2015)http://www.nature.com/bcj/journal/v5/n3/full/bcj201528a.html Accessed on November 13, 2015</ref> | [[Symptomatic]] [[patients]] with LPL are started on [[chemotherapy]] depending on the [[Staging (pathology)|stage]].<ref name="ADR">Waldenström's macroglobulinemia: prognosis and management. Blood Cancer Journal (2015)http://www.nature.com/bcj/journal/v5/n3/full/bcj201528a.html Accessed on November 13, 2015</ref> | ||
*Initial stage of LPL is | *Initial [[Staging (pathology)|stage]] of LPL is [[Association (statistics)|associated]] with: | ||
: | |||
:*[[Neuropathy]] | |||
:*[[ | :*[[Anemia]] or [[cytopenias]] | ||
:*[[ | :*Low-[[volume]] [[Nodal (protein)|nodal]] involvement | ||
:*[[Asymptomatic]] [[splenomegaly]] | |||
:* | |||
*Combinations of [[chemotherapy]] [[drugs]] that may be used include: | *Late [[Staging (pathology)|stage]] of LPL is [[Association (statistics)|associated]] with: | ||
**DRC – [[dexamethasone]] ([[Decadron]], [[Dexasone]]), [[rituximab]] ([[Rituxan]]) and [[cyclophosphamide]] | :*[[Adenopathy]] | ||
**BRD – [[bortezomib]] ([[Velcade]]) and [[rituximab]], with or without [[dexamethasone]] | :*[[Symptomatic]] [[splenomegaly]] | ||
**CVP – [[cyclophosphamide]], [[vincristine]] (Oncovin) and [[prednisone]] | :*[[Cytopenia|Cytopenias]] | ||
**R-CVP – CVP with [[rituximab]] | :*[[Hyperviscosity syndrome]] | ||
**[[Thalidomide]] ([[Thalomid]]) and [[rituximab]] | :*[[Neuropathy]] | ||
:*Constitutional [[symptoms]] | |||
*[[Men]] and [[Womens Pack|women]] with childbearing [[potential]] should receive [[counseling]] about the [[potential]] [[Effect size|effect]] of [[Treatments|treatment]] on their [[fertility]] and options for [[fertility]]-[[Preservative|preserving]] [[Measure (mathematics)|measures]]. | |||
*[[Chemotherapy]] [[drugs]] that may be used with or without [[prednisone]] include:<ref name="pmid190472842">{{cite journal| author=Dimopoulos MA, Gertz MA, Kastritis E, Garcia-Sanz R, Kimby EK, Leblond V et al.| title=Update on treatment recommendations from the Fourth International Workshop on Waldenstrom's Macroglobulinemia. | journal=J Clin Oncol | year= 2009 | volume= 27 | issue= 1 | pages= 120-6 | pmid=19047284 | doi=10.1200/JCO.2008.17.7865 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19047284 }}</ref> | |||
**[[Chlorambucil]] ([[Leukeran]]) | |||
**[[Fludarabine]] ([[Fludara]]) | |||
**[[Bendamustine]] ([[Treanda]]) | |||
**[[Cyclophosphamide]] ([[Cytoxan]], Procytox) | |||
*[[Combination therapy|Combinations]] of [[chemotherapy]] [[drugs]] that may be used include: | |||
**DRC – [[dexamethasone]] ([[Decadron]], [[Dexasone]]), [[rituximab]] ([[Rituxan]]) and [[cyclophosphamide]] | |||
**BRD – [[bortezomib]] ([[Velcade]]) and [[rituximab]], with or without [[dexamethasone]] | |||
**CVP – [[cyclophosphamide]], [[vincristine]] ([[Vincristine|Oncovin]]) and [[prednisone]] | |||
**R-CVP – CVP with [[rituximab]] | |||
**[[Thalidomide]] ([[Thalomid]]) and [[rituximab]] | |||
{| style="border: 0px; font-size: 90%; margin: 3px; width: 800px" | {| style="border: 0px; font-size: 90%; margin: 3px; width: 800px" | ||
| Line 54: | Line 96: | ||
! style="background: #4479BA; width: 300px;" | {{fontcolor|#FFF|Side effects}} | ! style="background: #4479BA; width: 300px;" | {{fontcolor|#FFF|Side effects}} | ||
|- | |- | ||
! style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" | | |||
'''[[CHOP-R regimen]]''' | '''[[CHOP-R regimen]]''' | ||
| style="padding: 5px 5px; background: #F5F5F5;" | | | style="padding: 5px 5px; background: #F5F5F5;" | | ||
| Line 69: | Line 111: | ||
*[[Mucositis]] | *[[Mucositis]] | ||
|- | |- | ||
! style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" | | |||
'''[[Ibrutinib]]''' | '''[[Ibrutinib]]''' | ||
| style="padding: 5px 5px; background: #F5F5F5;" | | | style="padding: 5px 5px; background: #F5F5F5;" | | ||
| Line 80: | Line 122: | ||
*[[Opportunistic infection]] | *[[Opportunistic infection]] | ||
|- | |- | ||
! style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" | | |||
'''[[Rituximab]]''' | '''[[Rituximab]]''' | ||
| style="padding: 5px 5px; background: #F5F5F5;" | | | style="padding: 5px 5px; background: #F5F5F5;" | | ||
| Line 86: | Line 128: | ||
| style="padding: 5px 5px; background: #F5F5F5;" | | | style="padding: 5px 5px; background: #F5F5F5;" | | ||
*[[Infusion-related reaction|Infusion related reaction]] | *[[Infusion-related reaction|Infusion related reaction]] | ||
*[[Hepatitis B]] reaction | *[[Hepatitis B]] [[reaction]] | ||
*Progressive multi-focal leukoencephaloptahy | *Progressive multi-focal leukoencephaloptahy | ||
|- | |- | ||
! style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" | | |||
'''FR regimen''' | '''FR regimen''' | ||
| style="padding: 5px 5px; background: #F5F5F5;" | | | style="padding: 5px 5px; background: #F5F5F5;" | | ||
| Line 99: | Line 141: | ||
*[[Pneumonia]] | *[[Pneumonia]] | ||
|- | |- | ||
! style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" | | |||
'''BDR regimen''' | '''BDR regimen''' | ||
| style="padding: 5px 5px; background: #F5F5F5;" | | | style="padding: 5px 5px; background: #F5F5F5;" | | ||
| Line 106: | Line 148: | ||
*[[Rituximab]] | *[[Rituximab]] | ||
| style="padding: 5px 5px; background: #F5F5F5;" | | | style="padding: 5px 5px; background: #F5F5F5;" | | ||
*[[Peripheral neuropathy]] - reversible in 61% of patients | *[[Peripheral neuropathy]] - [[Reversible cell|reversible]] in 61% of [[patients]] | ||
*[[Infections]] | *[[Infections]] | ||
|- | |- | ||
! style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" | | |||
'''DRC regimen''' | '''DRC regimen''' | ||
| style="padding: 5px 5px; background: #F5F5F5;" | | | style="padding: 5px 5px; background: #F5F5F5;" | | ||
| Line 118: | Line 160: | ||
*[[Neutropenia]] | *[[Neutropenia]] | ||
|- | |- | ||
! style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" | | |||
'''CR regimen''' | '''CR regimen''' | ||
| style="padding: 5px 5px; background: #F5F5F5;" | | | style="padding: 5px 5px; background: #F5F5F5;" | | ||
| Line 129: | Line 171: | ||
*[[Thrombocytopenia]] | *[[Thrombocytopenia]] | ||
|- | |- | ||
! style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" | | |||
'''IR regimen''' | '''IR regimen''' | ||
| style="padding: 5px 5px; background: #F5F5F5;" | | | style="padding: 5px 5px; background: #F5F5F5;" | | ||
| Line 144: | Line 186: | ||
{| | {| | ||
| | | | ||
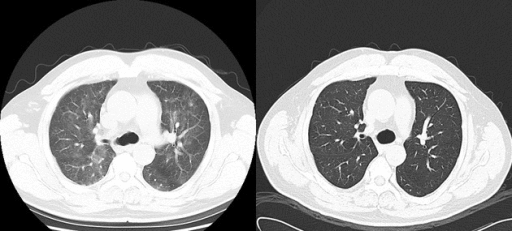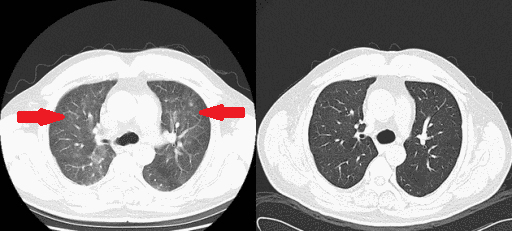
[[File: | [[File:Rituximab therapy gif.gif|thumb|250px|none|[[Interstitial pneumonitis]], post-[[rituximab]] therapy in a [[lymphoplasmacytic lymphoma]] patient. Helical computed tomographic scanning showed ground-glass shadowing in bilateral lungs before [[prednisone]] treatment and recovery at 1-week post-treatment. [https://openi.nlm.nih.gov/detailedresult.php?img=PMC4352371_ccr30003-0133-f1&query=waldenstrom+macroglobulinaemia&it=xg&req=4&npos=61 Source: Bai X. et al, Department of Hematology, Beijing Tiantan Hospital, Capital Medical University 6 Tiantan Xili Dongcheng District, Beijing, 100050, China.]]] | ||
|} | |} | ||
====Hyperviscosity syndrome:==== | ====Hyperviscosity syndrome:==== | ||
*[[Lymphoplasmacytic lymphoma]] complicated with [[hyperviscosity syndrome]] is a [[medical emergency]] and requires prompt treatment with [[plasmapheresis]].<ref name="ADR">Waldenström's macroglobulinemia: prognosis and management. Blood Cancer Journal (2015)http://www.nature.com/bcj/journal/v5/n3/full/bcj201528a.html Accessed on November 13, 2015</ref> | *[[Lymphoplasmacytic lymphoma]] [[Complication (medicine)|complicated]] with [[hyperviscosity syndrome]] is a [[medical emergency]] and requires [[prompt]] [[Treatments|treatment]] with [[plasmapheresis]].<ref name="ADR">Waldenström's macroglobulinemia: prognosis and management. Blood Cancer Journal (2015)http://www.nature.com/bcj/journal/v5/n3/full/bcj201528a.html Accessed on November 13, 2015</ref> | ||
*[[Plasmapheresis]] temporarily lowers [[IgM]] levels by removing some of the abnormal [[IgM]] from the [[blood]], which makes [[blood]] thinner. | *[[Plasmapheresis]] temporarily lowers [[IgM]] levels by removing some of the [[abnormal]] [[IgM]] from the [[blood]], which makes [[blood]] thinner. | ||
*[[Plasmapheresis]] is usually given until [[chemotherapy]] starts to work. | *[[Plasmapheresis]] is usually given until [[chemotherapy]] [[Starter (fermentation)|starts]] to [[Work (thermodynamics)|work]]. | ||
*[[Plasmapheresis]] is combined with [[chemotherapy]] to control the [[disease]] for a longer period of time. | *[[Plasmapheresis]] is [[Combination therapy|combined]] with [[chemotherapy]] to [[control]] the [[disease]] for a longer [[period]] of [[Time-series|time]]. | ||
*[[Plasmapheresis]] is also used in WM patients with [[hemolysis]]. | *[[Plasmapheresis]] is also used in [[Waldenström's macroglobulinemia|WM]] [[patients]] with [[hemolysis]]. | ||
===Initial treatment of Lymphoplasmacytic lymphoma:=== | ===Initial treatment of Lymphoplasmacytic lymphoma:=== | ||
{{familytree/start}} | {{familytree/start}} | ||
{{familytree | | | | | | | | | {{familytree |boxstyle=text-align: left;| | | | | | A01 | | | | | |A01= Does the [[patient]] has an [[indication]] for LPL treatment? | ||
*B symptoms (recurrent fever, night sweats, weight loss, fatigue) | *[[B symptoms]] (recurrent [[fever]], [[night sweats]], [[weight loss]], [[fatigue]]) | ||
*Hyperviscosity | *[[Hyperviscosity]] | ||
*Bulky/symptomatic lymphadenopathy | *Bulky/[[symptomatic]] [[lymphadenopathy]] | ||
*WM associated peripheral neuropathy | *[[Symptomatic]] [[hepatosplenomegaly]] | ||
*Cold agglutinin hemolytic anemia | *[[Symptomatic]] [[organomegaly]] or [[organ]]/[[tissue]] [[infiltration]] | ||
*Symptomatic cryoglobulinemia | *WM associated [[peripheral neuropathy]] | ||
*Immune hemolytic anemia and/or thrombocytopenia | *[[Cold agglutinin hemolytic anemia]] | ||
*LPL associated AL amyloidosis | *[[Symptomatic]] [[cryoglobulinemia]] | ||
*LPL associated nephropathy | *[[Immune hemolytic anemia]] and/or [[thrombocytopenia]] | ||
*Hemoglobin = or < 10g/dl | *LPL associated [[AL amyloidosis]] | ||
*Platelet count = or < 100 x 10'9/L | *LPL associated [[nephropathy]] | ||
{{familytree | *[[Hemoglobin]] = or < 10g/dl | ||
{{familytree | | | B01 | *[[Platelet]] count = or < 100 x 10'9/L}} | ||
{{familytree | | | |! | {{familytree | | | |,|-|-|^|-|-|.| | | }} | ||
{{familytree | | | C01 | {{familytree | | | B01 | | | | B02 | | |B01=Yes|B02=No}} | ||
{{familytree | |,|-|^|. | {{familytree | | | |!| | | | | |!| }} | ||
{{familytree | D01 | | D02 | {{familytree | | | C01 | | | | |!| |C01=Does the patient has [[symptoms]] associated with [[hyperviscosity]] such as: [[Oronasal]] [[bleeding]], [[blurred vision]], [[headaches]], [[dizziness]], [[paresthesias]], [[retinal vein engorgement]], [[flame-shaped hemorrhages]], [[papilledema]], [[stupor]] or [[coma]].|C02=asymptomatic/smoldering WM: Follow every 4-6 months with CBC and monoclonal protein levels}} | ||
{{familytree | | |,|-|^|.| | | |!| }} | |||
{{familytree | D01 | | D02 | |D03|D01=No|D02=Yes|D03=For smoldering/[[asymptomatic]] WM/LPL, just follow up every 4-6 months with [[CBC]] and [[monoclonal protein]] levels}} | |||
{{familytree | |!| | | |!| | | | | | | | | }} | {{familytree | |!| | | |!| | | | | | | | | }} | ||
{{familytree | E01 | | E02 |.| | | | | | |E01=Assess degree of symptom burden in WM/LPL pateint | {{familytree | E01 | | E02 |.| | | | | | |E01=Assess degree of [[symptom]] burden in WM/LPL [[pateint]]|E02=Consider emergent [[plasmapheresis]] for treatment of [[hyperviscosity]]}} | ||
{{Familytree |,|^|-|-|-|.| |!| | | }} | {{Familytree |,|^|-|-|-|.| |!| | | }} | ||
{{familytree | F01 | | F02 |!| | | | | | |F01=Low|F02=Moderate/High}} | {{familytree | F01 | | F02 |!| | | | | | |F01=Low|F02=Moderate/High}} | ||
{{Familytree | |!| | | |!| |!| | | | | | | }} | {{Familytree | |!| | | |!| |!| | | | | | | }} | ||
{{familytree | G01 | | G02 |'| | | |G01=Following are the 2 options for patients with low tumor burden with minimal symptoms: | {{familytree | G01 | | G02 |'| | | |G01=Following are the 2 options for [[patients]] with low [[tumor]] burden with minimal [[symptoms]]: | ||
*Single agent Rituximab | *Single agent [[Rituximab]] | ||
*Rituximab + chemotherapy as with high burden disease | *[[Rituximab]] + [[chemotherapy]] as with high burden [[disease]]|G02=Following 2 are the preferred [[regimens]] for [[moderate]]/[[severe]] [[symptoms]] or high [[tumor]] burden: | ||
*Bendamustine + rituximab | *[[Bendamustine]] + [[rituximab]] | ||
*Dexamethasone & rituximab + cyclophosphamide}} | *[[Dexamethasone]] & [[rituximab]] + [[cyclophosphamide]]}} | ||
{{familytree/end}} | {{familytree/end}} | ||
===Drug of choice for Bing-Neel Syndrome | ===Drug of choice for Bing-Neel Syndrome=== | ||
* Many recent studies have shown to be [[Ibrutinib]] (560mg), an oral Bruton's [[tyrosine kinase inhibitor]], with or without concurrent [[Rituximab]], as a [[drug]] of choice for treatment of [[Bing-Neel syndrome]]. It works by penetrating the [[blood brain barrier]].<ref name="pmid30228918">{{cite journal| author=O'Neil DS, Francescone MA, Khan K, Bachir A, O'Connor OA, Sawas A| title=A Case of Bing-Neel Syndrome Successfully Treated with Ibrutinib. | journal=Case Rep Hematol | year= 2018 | volume= 2018 | issue= | pages= 8573105 | pmid=30228918 | doi=10.1155/2018/8573105 | pmc=6136466 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=30228918 }} </ref><ref name="pmid27758817">{{cite journal| author=Minnema MC, Kimby E, D'Sa S, Fornecker LM, Poulain S, Snijders TJ et al.| title=Guideline for the diagnosis, treatment and response criteria for Bing-Neel syndrome. | journal=Haematologica | year= 2017 | volume= 102 | issue= 1 | pages= 43-51 | pmid=27758817 | doi=10.3324/haematol.2016.147728 | pmc=5210231 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=27758817 }} </ref><ref name="pmid30279255">{{cite journal| author=Tallant A, Selig D, Wanko SO, Roswarski J| title=First-line ibrutinib for Bing-Neel syndrome. | journal=BMJ Case Rep | year= 2018 | volume= 2018 | issue= | pages= | pmid=30279255 | doi=10.1136/bcr-2018-226102 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=30279255 }} </ref><ref name="pmid26689870">{{cite journal| author=Cabannes-Hamy A, Lemal R, Goldwirt L, Poulain S, Amorim S, Pérignon R et al.| title=Efficacy of ibrutinib in the treatment of Bing-Neel syndrome. | journal=Am J Hematol | year= 2016 | volume= 91 | issue= 3 | pages= E17-9 | pmid=26689870 | doi=10.1002/ajh.24279 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=26689870 }} </ref><ref name="pmid27409073">{{cite journal| author=Mason C, Savona S, Rini JN, Castillo JJ, Xu L, Hunter ZR et al.| title=Ibrutinib penetrates the blood brain barrier and shows efficacy in the therapy of Bing Neel syndrome. | journal=Br J Haematol | year= 2017 | volume= 179 | issue= 2 | pages= 339-341 | pmid=27409073 | doi=10.1111/bjh.14218 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=27409073 }} </ref> | * Many [[Recent changes|recent]] [[Study design|studies]] have shown to be [[Ibrutinib]] (560mg), an [[oral]] [[Bruton's tyrosine kinase]] [[tyrosine kinase inhibitor|inhibitor]], with or without [[Concurrent overlap|concurrent]] [[Rituximab]], as a [[drug]] of choice for the [[Treatments|treatment]] of [[Bing-Neel syndrome]]. It [[Work function|works]] by [[Penetrance|penetrating]] the [[blood brain barrier]].<ref name="pmid30228918">{{cite journal| author=O'Neil DS, Francescone MA, Khan K, Bachir A, O'Connor OA, Sawas A| title=A Case of Bing-Neel Syndrome Successfully Treated with Ibrutinib. | journal=Case Rep Hematol | year= 2018 | volume= 2018 | issue= | pages= 8573105 | pmid=30228918 | doi=10.1155/2018/8573105 | pmc=6136466 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=30228918 }} </ref><ref name="pmid27758817">{{cite journal| author=Minnema MC, Kimby E, D'Sa S, Fornecker LM, Poulain S, Snijders TJ et al.| title=Guideline for the diagnosis, treatment and response criteria for Bing-Neel syndrome. | journal=Haematologica | year= 2017 | volume= 102 | issue= 1 | pages= 43-51 | pmid=27758817 | doi=10.3324/haematol.2016.147728 | pmc=5210231 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=27758817 }} </ref><ref name="pmid30279255">{{cite journal| author=Tallant A, Selig D, Wanko SO, Roswarski J| title=First-line ibrutinib for Bing-Neel syndrome. | journal=BMJ Case Rep | year= 2018 | volume= 2018 | issue= | pages= | pmid=30279255 | doi=10.1136/bcr-2018-226102 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=30279255 }} </ref><ref name="pmid26689870">{{cite journal| author=Cabannes-Hamy A, Lemal R, Goldwirt L, Poulain S, Amorim S, Pérignon R et al.| title=Efficacy of ibrutinib in the treatment of Bing-Neel syndrome. | journal=Am J Hematol | year= 2016 | volume= 91 | issue= 3 | pages= E17-9 | pmid=26689870 | doi=10.1002/ajh.24279 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=26689870 }} </ref><ref name="pmid27409073">{{cite journal| author=Mason C, Savona S, Rini JN, Castillo JJ, Xu L, Hunter ZR et al.| title=Ibrutinib penetrates the blood brain barrier and shows efficacy in the therapy of Bing Neel syndrome. | journal=Br J Haematol | year= 2017 | volume= 179 | issue= 2 | pages= 339-341 | pmid=27409073 | doi=10.1111/bjh.14218 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=27409073 }} </ref> | ||
* One or more of the following [[treatments]] can be given for [[lymphoplasmacytic lymphoma]]. | |||
* | =====Targeted therapy===== | ||
*[[Targeted therapy]] uses [[drugs]] to [[Targeted therapy|target]] [[Specific activity|specific]] [[molecules]] (such as [[proteins]]) on the [[Surface anatomy|surface]] of [[cancer cells]]. These [[molecules]] help send [[Signals (biology)|signals]] that tell [[Cells (biology)|cells]] to [[Growth|grow]] or divide. By [[Targeted therapy|targeting]] these [[molecules]], the [[drugs]] stop the [[growth]] and [[Spread of the cancer|spread of cancer]] [[cancer cells|cells]] while [[Limiting factor|limiting]] harm to [[normal]] [[cells]].<ref name="pmid260029632">{{cite journal| author=Treon SP| title=How I treat Waldenström macroglobulinemia. | journal=Blood | year= 2015 | volume= 126 | issue= 6 | pages= 721-32 | pmid=26002963 | doi=10.1182/blood-2015-01-553974 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=26002963 }}</ref> | |||
*[[Targeted therapy]] [[drugs]] used alone or in [[Combination therapy|combination]] to [[Treatments|treat]] [[lymphoplasmacytic lymphoma]] include [[rituximab]], [[bortezomib]] and [[ibrutinib]] (Imbruvica).<ref name="pmid26002963">{{cite journal| author=Treon SP| title=How I treat Waldenström macroglobulinemia. | journal=Blood | year= 2015 | volume= 126 | issue= 6 | pages= 721-32 | pmid=26002963 | doi=10.1182/blood-2015-01-553974 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=26002963 }}</ref><ref name="pmid190472843">{{cite journal| author=Dimopoulos MA, Gertz MA, Kastritis E, Garcia-Sanz R, Kimby EK, Leblond V et al.| title=Update on treatment recommendations from the Fourth International Workshop on Waldenstrom's Macroglobulinemia. | journal=J Clin Oncol | year= 2009 | volume= 27 | issue= 1 | pages= 120-6 | pmid=19047284 | doi=10.1200/JCO.2008.17.7865 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19047284 }}</ref> | |||
===== | =====Immunotherapy===== | ||
*[[ | *[[Immunotherapy]] works by [[Stimulant|stimulating]], [[boosting]], [[Restoration device|restoring]] or [[Acting out|acting]] like the [[Body|body’s]] [[immune system]] to create a [[Response element|response]] against [[cancer cells]]. [[Immunomodulatory]] [[drugs]] are a type of [[immunotherapy]] that [[Interference|interferes]] with the [[growth]] and [[Division (biology)|division]] of [[cancer cells]]. | ||
*[[ | *[[Thalidomide]] is a type of [[immunomodulatory]] [[drug]] that may be used to [[Treatments|treat]] [[lymphoplasmacytic lymphoma]].<ref name="pmid19047284">{{cite journal| author=Dimopoulos MA, Gertz MA, Kastritis E, Garcia-Sanz R, Kimby EK, Leblond V et al.| title=Update on treatment recommendations from the Fourth International Workshop on Waldenstrom's Macroglobulinemia. | journal=J Clin Oncol | year= 2009 | volume= 27 | issue= 1 | pages= 120-6 | pmid=19047284 | doi=10.1200/JCO.2008.17.7865 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19047284 }}</ref> | ||
===== | =====Radiation therapy===== | ||
* In some [[rare]] [[Case-based reasoning|cases]], [[external beam radiation therapy]] may be required to [[Treatments|treat]] [[Lymphoplasmacytic lymphoma|LPL]] that [[Development|develops]] outside of the [[lymphatic system]] (called extralymphatic [[disease]]). | |||
In some rare cases, [[external beam radiation therapy]] may be required to treat LPL that develops outside of the [[lymphatic system]] (called extralymphatic [[disease]]). | |||
==References== | ==References== | ||
| Line 207: | Line 252: | ||
{{WH}} | {{WH}} | ||
{{WS}} | {{WS}} | ||
[[Category: | [[Category:Disease]] | ||
[[Category:Blood]] | |||
[[Category:Hematology]] | |||
Latest revision as of 13:56, 31 October 2019
|
Lymphoplasmacytic lymphoma Microchapters |
|
Differentiating Lymphoplasmacytic Lymphoma from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Lymphoplasmacytic lymphoma medical therapy On the Web |
|
American Roentgen Ray Society Images of Lymphoplasmacytic lymphoma medical therapy |
|
Risk calculators and risk factors for Lymphoplasmacytic lymphoma medical therapy |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sara Mohsin, M.D.[2]
Overview
Risk stratification determines the protocol of management used for lymphoplasmacytic lymphoma. There is no treatment for asymptomatic lymphoplasmacytic lymphoma. The mainstay of treatment for symptomatic lymphoplasmacytic lymphoma is Rituximab +/- Chemotherapy. Hyperviscosity syndrome is a medical emergency and requires prompt treatment with plasmapheresis. Drug of choice for the treatment of bing-neel syndrome is Ibrutinib with or without concurrent rituximab. Other treatment options include targeted therapy, immunotherapy and radiation therapy.
Medical Therapy
There's no cure for LPL with current therapies. Instead, the treatment goals are to control symptoms and prevent end-organ damage, while maximizing quality of life. There is no standard therapy for the treatment of LPL. While various drugs and combinations have demonstrated to have provided clinical benefit, hence, there are several different options for treating lymphoplasmacytic lymphoma depending on stage of the disease:[1]
| Patient's condition/parameters | How to proceed accordingly |
|---|---|
|
Observation |
|
|
|
Hperviscosity present:
Hyperviscosity absent:
|
|
Consider clinical trial + stem cell transplant in selected patients:
|
Watchful waiting/active surveillance for asymptomatic patients with LPL
There is no treatment for asymptomatic patients with LPL. As LPL develops slowly and may not need to be treated right away, it is monitored by healthcare team every 3-6 months which is known as watchful waiting/active surveillance and treatment is started when symptoms appear, such as hyperviscosity syndrome, or there are signs that the disease is progressing more quickly.[2] Active surveillance includes monitoring of the following laboratory parameters:
- Complete blood count (CBC) with differential
- Complete metabolic panel (CMP)
- Immunoglobulin levels in the serum (quantitative)
- Serum protein electrophoresis
Symptomatic patients with LPL
Symptomatic patients with LPL are started on chemotherapy depending on the stage.[3]
- Initial stage of LPL is associated with:
- Neuropathy
- Anemia or cytopenias
- Low-volume nodal involvement
- Asymptomatic splenomegaly
- Late stage of LPL is associated with:
- Adenopathy
- Symptomatic splenomegaly
- Cytopenias
- Hyperviscosity syndrome
- Neuropathy
- Constitutional symptoms
- Men and women with childbearing potential should receive counseling about the potential effect of treatment on their fertility and options for fertility-preserving measures.
- Chemotherapy drugs that may be used with or without prednisone include:[4]
- Chlorambucil (Leukeran)
- Fludarabine (Fludara)
- Bendamustine (Treanda)
- Cyclophosphamide (Cytoxan, Procytox)
- Combinations of chemotherapy drugs that may be used include:
- DRC – dexamethasone (Decadron, Dexasone), rituximab (Rituxan) and cyclophosphamide
- BRD – bortezomib (Velcade) and rituximab, with or without dexamethasone
- CVP – cyclophosphamide, vincristine (Oncovin) and prednisone
- R-CVP – CVP with rituximab
- Thalidomide (Thalomid) and rituximab
| Treatment Regimen[3]
|
Drugs | Side effects |
|---|---|---|
| ||
|
FR regimen |
||
|
BDR regimen |
| |
|
DRC regimen |
||
|
CR regimen |
||
|
IR regimen |
 |
Hyperviscosity syndrome:
- Lymphoplasmacytic lymphoma complicated with hyperviscosity syndrome is a medical emergency and requires prompt treatment with plasmapheresis.[3]
- Plasmapheresis temporarily lowers IgM levels by removing some of the abnormal IgM from the blood, which makes blood thinner.
- Plasmapheresis is usually given until chemotherapy starts to work.
- Plasmapheresis is combined with chemotherapy to control the disease for a longer period of time.
- Plasmapheresis is also used in WM patients with hemolysis.
Initial treatment of Lymphoplasmacytic lymphoma:
Drug of choice for Bing-Neel Syndrome
- Many recent studies have shown to be Ibrutinib (560mg), an oral Bruton's tyrosine kinase inhibitor, with or without concurrent Rituximab, as a drug of choice for the treatment of Bing-Neel syndrome. It works by penetrating the blood brain barrier.[5][6][7][8][9]
- One or more of the following treatments can be given for lymphoplasmacytic lymphoma.
Targeted therapy
- Targeted therapy uses drugs to target specific molecules (such as proteins) on the surface of cancer cells. These molecules help send signals that tell cells to grow or divide. By targeting these molecules, the drugs stop the growth and spread of cancer cells while limiting harm to normal cells.[10]
- Targeted therapy drugs used alone or in combination to treat lymphoplasmacytic lymphoma include rituximab, bortezomib and ibrutinib (Imbruvica).[11][12]
Immunotherapy
- Immunotherapy works by stimulating, boosting, restoring or acting like the body’s immune system to create a response against cancer cells. Immunomodulatory drugs are a type of immunotherapy that interferes with the growth and division of cancer cells.
- Thalidomide is a type of immunomodulatory drug that may be used to treat lymphoplasmacytic lymphoma.[13]
Radiation therapy
- In some rare cases, external beam radiation therapy may be required to treat LPL that develops outside of the lymphatic system (called extralymphatic disease).
References
- ↑ Lymphoplasmacytic lymphoma. Canadian Cancer Society 2015. http://www.cancer.ca/en/cancer-information/cancer-type/non-hodgkin-lymphoma/non-hodgkin-lymphoma/types-of-nhl/lymphoplasmacytic-lymphoma/?region=ab Accessed on November 6 2015
- ↑ Waldenström's macroglobulinemia. Patient (2015)http://patient.info/doctor/waldenstroms-macroglobulinaemia-pro Accessed on November 10, 2015
- ↑ 3.0 3.1 3.2 Waldenström's macroglobulinemia: prognosis and management. Blood Cancer Journal (2015)http://www.nature.com/bcj/journal/v5/n3/full/bcj201528a.html Accessed on November 13, 2015
- ↑ Dimopoulos MA, Gertz MA, Kastritis E, Garcia-Sanz R, Kimby EK, Leblond V; et al. (2009). "Update on treatment recommendations from the Fourth International Workshop on Waldenstrom's Macroglobulinemia". J Clin Oncol. 27 (1): 120–6. doi:10.1200/JCO.2008.17.7865. PMID 19047284.
- ↑ O'Neil DS, Francescone MA, Khan K, Bachir A, O'Connor OA, Sawas A (2018). "A Case of Bing-Neel Syndrome Successfully Treated with Ibrutinib". Case Rep Hematol. 2018: 8573105. doi:10.1155/2018/8573105. PMC 6136466. PMID 30228918.
- ↑ Minnema MC, Kimby E, D'Sa S, Fornecker LM, Poulain S, Snijders TJ; et al. (2017). "Guideline for the diagnosis, treatment and response criteria for Bing-Neel syndrome". Haematologica. 102 (1): 43–51. doi:10.3324/haematol.2016.147728. PMC 5210231. PMID 27758817.
- ↑ Tallant A, Selig D, Wanko SO, Roswarski J (2018). "First-line ibrutinib for Bing-Neel syndrome". BMJ Case Rep. 2018. doi:10.1136/bcr-2018-226102. PMID 30279255.
- ↑ Cabannes-Hamy A, Lemal R, Goldwirt L, Poulain S, Amorim S, Pérignon R; et al. (2016). "Efficacy of ibrutinib in the treatment of Bing-Neel syndrome". Am J Hematol. 91 (3): E17–9. doi:10.1002/ajh.24279. PMID 26689870.
- ↑ Mason C, Savona S, Rini JN, Castillo JJ, Xu L, Hunter ZR; et al. (2017). "Ibrutinib penetrates the blood brain barrier and shows efficacy in the therapy of Bing Neel syndrome". Br J Haematol. 179 (2): 339–341. doi:10.1111/bjh.14218. PMID 27409073.
- ↑ Treon SP (2015). "How I treat Waldenström macroglobulinemia". Blood. 126 (6): 721–32. doi:10.1182/blood-2015-01-553974. PMID 26002963.
- ↑ Treon SP (2015). "How I treat Waldenström macroglobulinemia". Blood. 126 (6): 721–32. doi:10.1182/blood-2015-01-553974. PMID 26002963.
- ↑ Dimopoulos MA, Gertz MA, Kastritis E, Garcia-Sanz R, Kimby EK, Leblond V; et al. (2009). "Update on treatment recommendations from the Fourth International Workshop on Waldenstrom's Macroglobulinemia". J Clin Oncol. 27 (1): 120–6. doi:10.1200/JCO.2008.17.7865. PMID 19047284.
- ↑ Dimopoulos MA, Gertz MA, Kastritis E, Garcia-Sanz R, Kimby EK, Leblond V; et al. (2009). "Update on treatment recommendations from the Fourth International Workshop on Waldenstrom's Macroglobulinemia". J Clin Oncol. 27 (1): 120–6. doi:10.1200/JCO.2008.17.7865. PMID 19047284.