Lorlatinib
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Uma Maveli[2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: SERIOUS CENTRAL NERVOUS SYSTEM EFFECTS
See full prescribing information for complete Boxed Warning.
Broad-spectrum of Central Nervous system side effects with the use of Lorlatinib
|
Overview
Lorlatinib is a Antineoplastic Agents that is FDA approved for the treatment of The treatment of patients with lung cancer
- Should not be given to patients experiencing life threatening episodes
- In other words, Revefenacin should not be used as a rescue drug
- Discontinue the drug if patients appears to suffer from paradoxical bronchospasm or hypersensitivity reactions. There is a Black Box Warning for this drug as shown here. Common adverse reactions include Edema, Peripheral Neuropathy, Cognitive Effects, Dyspnea (shortness of breath), fatigue, weight gain, arthralgia, mood effects, diarrhea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Lorlatinib is indicated for:
- The treatment of patients with anaplastic lymphoma kinase- aggressive, wide-spread lung cancer
- It is indicated for with:
- Crizonitib and another ALK inhibitor, or Alectinib, the first therapy for metastatic disease, or Ceritinib as the first ALK inhibitor for this type of disease
Limitations of Use
- Revefenacin delivered via jet nebulizer can result in "longer administration time, variability in residual volume and particle size, daily cleaning requirements, limited portability, and need for device assembly"
- The benefits may outweigh this because some patients are required to use nebulizers
Recommended Vaccination and Prophylaxis
Recommended Weight-Based Dosage Regimen - OCPD
Recommended Weight-Based Dosage Regimen - aHUS
Dosing Considerations
- Some dose reductions if the patients have adverse reactions include:
- Swallow Lorlatinib orally in a recommended dosage of 75 mg once daily
- If there is need for more reduction, take Lorlatinib 50 mg once daily orally
Preparation of Lorlatinib
Administration of Lorlatinib
- It is recommended that the patient take 100 mg of Lorlatinib, no matter if they have or have not eaten
- The tablets are meant to be swallowed whole: it is unacceptable to chew, break, split tablets because it will not have the same effect and it could be dangerous
- Make sure to not to take them if they are broken, cracked, not in packaging etc
- It is important that the patient takes the medication the same time each day
- The patient may take the forgotten dose if it is more that 4 hours from their next dose
- It is really important that they do not take multiple doses at once
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Lorlatinib Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding Lorlatinib Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Lorlatinib FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Lorlatinib Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding Lorlatinib Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
- It is crucial to withhold ingesting Lorlatinib for three plasma half lives for patients taking strong CYP3A inducers
- Mixing them together could lead to serious hepatotoxicity or chemical-driven liver damage
Warnings
|
WARNING: SERIOUS CENTRAL NERVOUS SYSTEM EFFECTS
See full prescribing information for complete Boxed Warning.
Broad-spectrum of Central Nervous system side effects with the use of Lorlatinib
|
Serious Central Nervous System Effects
- Seizures, Hallucinations, changes in mood, sleep, mental health are all examples of the side effects
- Reduce the dosage of Lorlatinib depending on the severity of the side effect
- For patients requiring first time reduction, give them 75 mg daily through mouth
- For patients requiring second time reduction, give them 50 mg daily through mouth
- About 54% of patients acquiring this drug may experience Central Nervous system side effects listed above
Hyperlipidemia
- Patients will be monitored for the first 1-2 months ingesting Lorbrena, and followed up on after the initial period
- Patients may experience an increase in serum cholesterol and triglycerides
- About 7% of patients in the Study B7461001 required to discontinue the drug for a short period of time, and another 3% of the patients required a dose reduction
- In the same study, 80% of the patients required to instigate lipid-lowering medications because they were not responding to reduction and temporary pause
- This may have required a period of adjustment for 21 days to get accustomed to the lipid-lowering medications
Monitoring Disease Manifestations after ULTOMIRIS Discontinuation
Treatment Discontinuation for PNH
- After discontinuing treatment with ULTOMIRIS, closely monitor for signs and symptoms of hemolysis, identified by elevated LDH along with sudden decrease in PNH clone size or hemoglobin, or re-appearance of symptoms such as fatigue, hemoglobinuria, abdominal pain, shortness of breath (dyspnea), major adverse vascular event (including thrombosis), dysphagia, or erectile dysfunction.
- Monitor any patient who discontinues ULTOMIRIS for at least 16 weeks to detect hemolysis and other reactions.
- If signs and symptoms of hemolysis occur after discontinuation, including elevated LDH, consider restarting treatment with ULTOMIRIS.
Treatment Discontinuation for aHUS
- ULTOMIRIS treatment of aHUS should be a minimum duration of 6 months.
- Patients should be monitored for clinical symptoms and laboratory signs of TMA complications for at least 12 months.
- TMA complications post-discontinuation can be identified if any of the following is observed:
- Changes in mental status, seizures, angina, dyspnea, thrombosis or increasing blood pressure
- At least two of the following laboratory signs observed concurrently and results should be confirmed by a second measurement 28 days apart with no interruption:
- A decrease in platelet count of 25% or more as compared to either baseline or to peak platelet count during ULTOMIRIS treatment;
- An increase in serum creatinine of 25% or more as compared to baseline or to nadir during ULTOMIRIS treatment
- An increase in serum LDH of 25% or more as compared to baseline or to nadir during ULTOMIRIS treatment
- If TMA complications occur after ULTOMIRIS discontinuation, consider reinitiation of ULTOMIRIS treatment or appropriate organ-specific supportive measures.
Infusion Reactions
- In clinical trials, 5 out of 296 patients treated with ULTOMIRIS experienced infusion reactions (lower back pain, drop in blood pressure, infusion-related pain, elevation in blood pressure and limb discomfort) during ULTOMIRIS administration.
- Interrupt ULTOMIRIS infusion and institute appropriate supportive measures if signs of cardiovascular instability or respiratory compromise occur.
Adverse Reactions
Clinical Trials Experience
Paroxysmal Nocturnal Hemoglobinuria (PNH)
- Most common adverse reactions in patients with PNH (incidence ≥10%) were upper respiratory tract infection and headache
Atypical Hemolytic Uremic Syndrome (aHUS)
- Most common adverse reactions in patients with aHUS (incidence ≥20%) were upper respiratory tract infection, diarrhea, nausea, vomiting, headache, hypertension and pyrexia
Immunogenicity
- The immunogenicity of ravulizumab-cwvz has been evaluated using an enzyme-linked immunosorbent assay (ELISA) for the detection of binding anti-ravulizumab-cwvz antibodies. For patients whose sera tested positive in the screening immunoassay, an in vitro biological assay was performed to detect neutralizing antibodies.
- In clinical studies, treatment-emergent antibodies to ravulizumab-cwvz were detected in 1 of 206 (0.5%) patients with PNH and 1 of 71 (1.4%) patients with aHUS.
- No apparent correlation of antibody development to altered pharmacokinetic profile, clinical response, or adverse events was observed.
Postmarketing Experience
There is limited information regarding Yupelri Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Yupelri Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There are no available data on Revefencain use in pregnant women to inform a drugassociated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Lorlatinib in women who are pregnant.
Labor and Delivery
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Nursing Mothers
There is no FDA guidance on the use of Revefenacin with respect to nursing.
Pediatric Use
The safety and efficacy of Revefenacin for the treatment of PNH in pediatric patients have not been established.
Geriatic Use
Clinical studies of Ultomiris did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
Gender
There is no FDA guidance on the use of Revefenacin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Revefenacin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Revefenacin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Revefenacin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Revefenacin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Revefenacin in patients who are immunocompromised.
Administration and Monitoring
Administration
- After intravenous administration of revefenacin, the reported volume of distribution is 218 L which suggests an extensive distribution to the tissues
Monitoring
- Monitor patients for early signs and symptoms of meningococcal infection.
- Monitor the patient for at least one hour following completion of the infusion for signs or symptoms of an infusion reaction.
- After discontinuing treatment with ULTOMIRIS, patients should be monitored for clinical symptoms and laboratory signs of TMA complications for at least 12 months.
- Clinical symptoms of TMA include changes in mental status, seizures, angina, dyspnea, thrombosis or increasing blood pressure.
IV Compatibility
There is limited information regarding the compatibility of Lorlatinib and IV administrations.
Overdosage
Common signs and symptoms of overdosage of Revefenacin:
- nausea, vomiting, dizziness, lightheadedness, blurred vision, increased intraocular pressure, obstipation and difficulties in voiding
- If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Lorlatinib
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | 55768.94 Da |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
- Ravulizumab-cwvz is a terminal complement inhibitor that specifically binds to the complement protein C5 with high affinity, thereby inhibiting its cleavage to C5a (the proinflammatory anaphylatoxin) and C5b (the initiating subunit of the terminal complement complex [C5b-9]) and preventing the generation of the terminal complement complex C5b9.
- ULTOMIRIS inhibits terminal complement-mediated intravascular hemolysis in patients with PNH and complement-mediated thrombotic microangiopathy (TMA) in patients with aHUS.
Structure
There is limited information regarding Ultomiris Structure in the drug label.
Pharmacodynamics
- The extent and duration of the pharmacodynamic response in patients with PNH and aHUS were exposure-dependent for ULTOMIRIS. Free C5 levels of <0.5 mcg/mL were correlated with maximal intravascular hemolysis control and complete terminal complement inhibition in patients with PNH.
- Complete terminal complement inhibition following initiation of ULTOMIRIS treatment led to normalization of serum LDH by week 4 in complement-inhibitor naïve patients with PNH, and maintained LDH normalization in patients previously treated with eculizumab with PNH.
Pharmacokinetics
Ravulizumab-cwvz pharmacokinetics increase proportionally over a dose range of 200 to 5400 mg.
Distribution
The reported volume of distribution is 218 L which suggests an extensive distribution to the tissues
Elimination
There are two phases of elimination: Kinetics Elimination: rapid declining plasma concentration followed by slow bi-exponential elimination. Renal Elimination: the amount excreted in urine is the unchanged drug, <0.2% of the administered dose. Following the IV administration, 54% of dose is recovered in feces and 27* in urine
Specific Populations
No clinically significant differences in the pharmacokinetics of ravulizumab-cwvz were observed based on sex, age (10 months to 83 years), race, hepatic impairment, or any degree of renal impairment, including patients with proteinuria or receiving dialysis. Body weight was a clinically significant covariate on the pharmacokinetics of ravulizumab-cwvz.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- No animal studies were performed to evaluate the effects of ravulizumab-cwvz on carcinogenesis, or mutagenesis.
- Effects of ravulizumab-cwvz upon fertility have not been studied in animals.
- Intravenous injections of male and female mice with a murine anti-C5 antibody at up to 0.8-2.2 times the equivalent of the clinical dose of ULTOMIRIS had no adverse effects on mating or fertility.
Clinical Studies
Anaplastic Lymphoma Kinase(COPD)
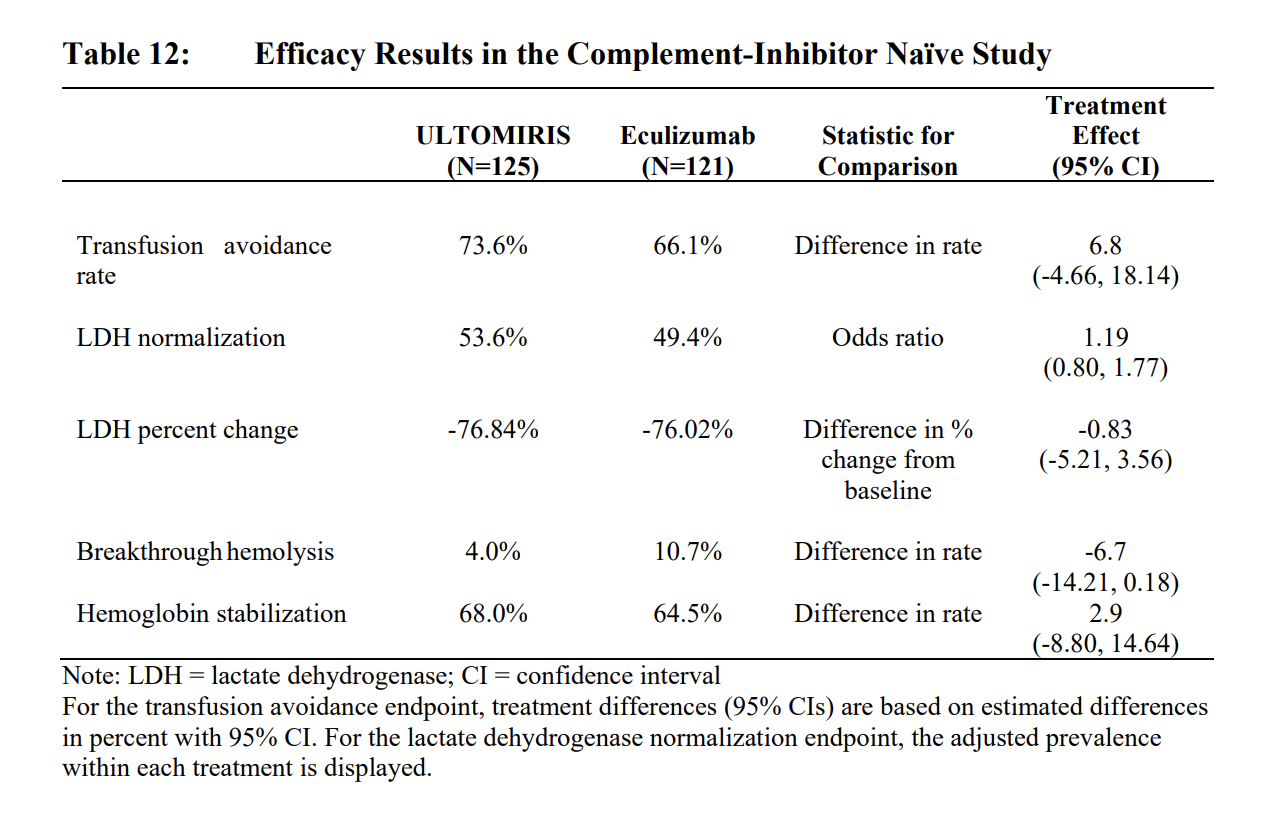
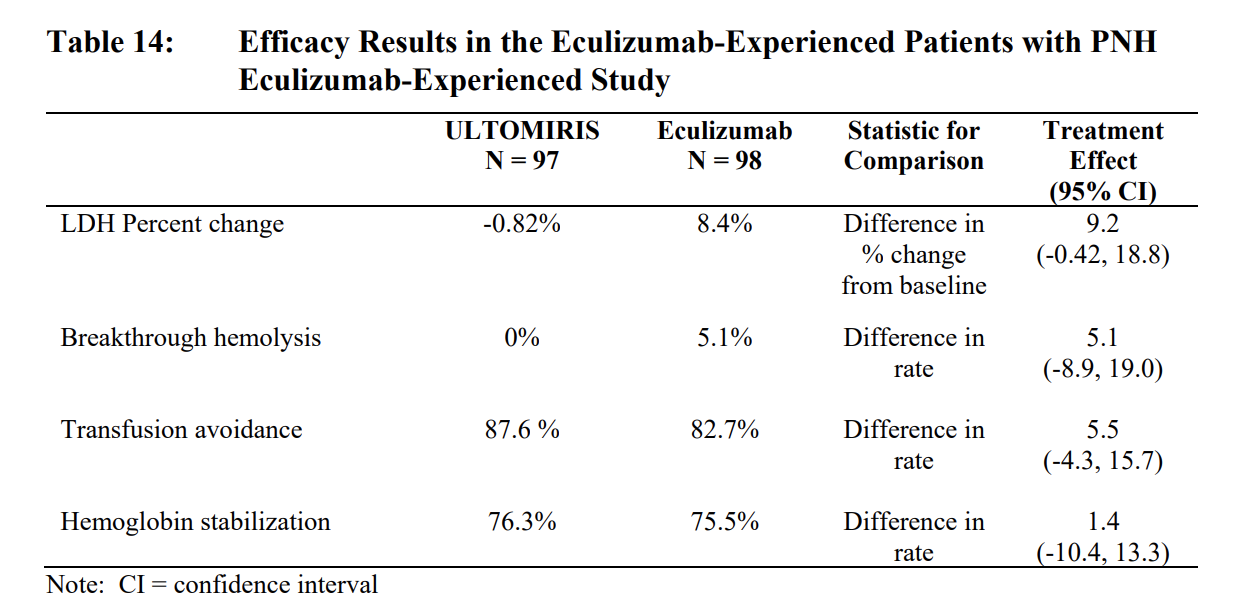
- The safety and efficacy of ULTOMIRIS in patients with PNH was assessed in two openlabel, randomized, active-controlled, non-inferiority Phase 3 studies: PNH Study 301 and PNH Study 302.
- ULTOMIRIS was dosed intravenously in accordance with the weightbased dosing (4 infusions of ULTOMIRIS over 26 weeks). Eculizumab was administered on Days 1, 8, 15, and 22, followed by maintenance treatment with 900 mg of eculizumab on Day 29 and every 2 weeks (q2w) thereafter for a total of 26 weeks of treatment, according to the approved dosing regimen of eculizumab which was the standard-of-care for PNH at the time of studies.
- Patients were vaccinated against meningococcal infection prior to or at the time of initiating treatment with ULTOMIRIS or eculizumab, or received prophylactic treatment with appropriate antibiotics until 2 weeks after vaccination.
- There was no observable difference in fatigue between ULTOMIRIS and eculizumab after 26 weeks of treatment compared to baseline as measured by the FACIT-fatigue instrument. Patient-reported fatigue may be an under-or over-estimation, because patients were not blinded to treatment assignment.
ALK Study 301 [ALXN1210-PNH-301; NCT02946463]
- enrolled patients with PNH who were complement inhibitor naïve and had active hemolysis
- 26-week, multicenter, open-label, randomized, active-controlled, non-inferiority Phase 3 study conducted in 246 patients naïve to complement inhibitor treatment prior to study entry
- Patients with PNH with flow cytometric confirmation of at least 5% PNH cells were randomized 1:1 to either ULTOMIRIS or eculizumab.
ALK Study 302 [ALXN1210-PNH-302; NCT03056040]
- Enrolled patients with PNH who were clinically stable after having been treated with eculizumab for at least the past 6 months.
- Patients who demonstrated clinically stable disease after being treated with eculizumab for at least the prior 6 months were randomized 1:1 to either continue eculizumab or to switch to ULTOMIRIS.
Study in Adult Patients with Anaplastic Lymphoma Kinase [ALXN1210-aHUS-311; NCT02949128]
- Conducted in patients who were naïve to complement inhibitor treatment prior to study entry.
- The study consisted of a 26-week Initial Evaluation Period and patients were allowed to enter an extension period for up to 4.5 years.
- A total of 56 patients with aHUS were evaluated for efficacy.
- Ninety-three percent of patients had extra-renal signs (cardiovascular, pulmonary, central nervous system, gastrointestinal, skin, skeletal muscle) or symptoms of aHUS at baseline.
- At baseline,71.4% (n = 40) of patients had Stage 5 chronic kidney disease (CKD). Fourteen percent had a medical history of kidney transplant and 51.8% were on dialysis at study entry. Eight patients entered the study with evidence of TMA for > 3 days after childbirth (ie, postpartum).
- Renal function, as measured by eGFR, was improved or maintained during ULTOMIRIS therapy.

Study in Pediatric Patients with Anaplastic Lymphoma Kinase[ALXN1210-aHUS-312; NCT03131219]
- There is limited information regarding Lorlatinib Studies in Pediatric Patients
How Supplied
- It is supplied in a child-resistant vial that contains 25 or 100 mg worth of tablets. There will be 30 tablets in the bottle. Some of the inactive ingredients in the tablet include microcrystalline cellulose, sodium starch glycolate, and magnesium stearate.
- The 25 mg bottles will contain tablets that appear as 8mm round, tan-colored, film-coated, and has 25 on one side and LLN on the other side
- The 100 mg bottles will contain tablets that appear as 8.5 by 17 mm round, lavender-colored, film-coated, contains Pfizer on on side, and 100 and LLn on the other
Storage
- Lorbrena is stored in a temperature of 20-25 degrees Celsius (68-77 degrees Fahrenheit)
- It is allowed to be exposed to temperatures from 15-30 degrees Celsius for some time
Images
Drug Images
{{#ask: Page Name::Lorlatinib |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Lorlatinib |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Hepatotoxicity
- Patients should be aware that utilizing Lobrena concurrent to CYP3A inducers can lead to serious cases of hepatotoxicity
- Patients should check with a doctor to confirm the compatibility of certain medications with Lobrena
Other Infections
Discontinuation
- Patients who express Paradoxical Bronchospasm, which means breathing or wheezing will worsen, should discontinue Revefenacin and initiate therapy with another agent
Infusion reactions
- There is limited information on Lobrena infusion reactions
Precautions with Alcohol
Alcohol-Lorlatinib interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
Lorbrena
Look-Alike Drug Names
There is limited information regarding Lorlatinib Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.