Listeria monocytogenes: Difference between revisions
Shanshan Cen (talk | contribs) No edit summary |
Shanshan Cen (talk | contribs) |
||
| Line 55: | Line 55: | ||
==Treatment== | ==Treatment== | ||
===Antimicrobial regimen=== | ===Antimicrobial regimen=== | ||
* Meningitis | |||
:* Preferred regimen: [[Ampicillin]] 2g IV q4-6h {{withorwithout}} [[Gentamicin]] 1.7 mg/kg IV q8h for more than 3 weeks | |||
:* Alternative regimen: [[TMP-SMX]] 3-5 mg/kg (trimethoprim) q6h IV for more than 3 weeks | |||
* Bacteremia | |||
:* Preferred regimen: [[Ampicillin]] 2g IV q4-6h {{withorwithout}} [[Gentamicin]] 1.7 mg/kg IV q8h for 2 weeks | |||
:* Alternative regimen: [[TMP-SMX]] 3-5 mg/kg (trimethoprim) q6h IV for 2 weeks | |||
* Brain abscess or rhomboencephalitis | |||
:* Preferred regimen: [[Ampicillin]] 2g IV q4-6h {{withorwithout}} [[Gentamicin]] 1.7 mg/kg IV q8h for 4-6 weeks | |||
:* Alternative regimen: [[TMP-SMX]] 3-5 mg/kg (trimethoprim) q6h IV for 4-6 weeks | |||
* Gastroenteritis | |||
==References== | ==References== | ||
Revision as of 13:23, 29 June 2015
|
Listeriosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Listeria monocytogenes On the Web |
|
American Roentgen Ray Society Images of Listeria monocytogenes |
|
Risk calculators and risk factors for Listeria monocytogenes |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: João André Alves Silva, M.D. [2]
Overview
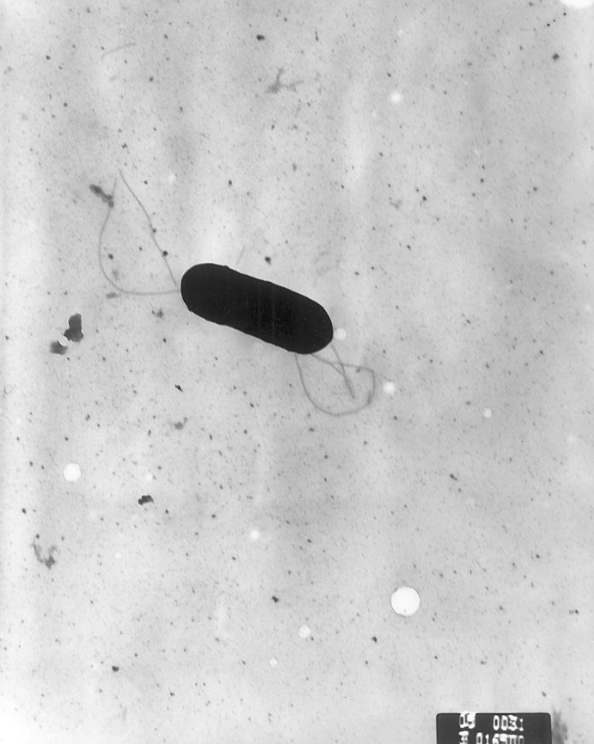
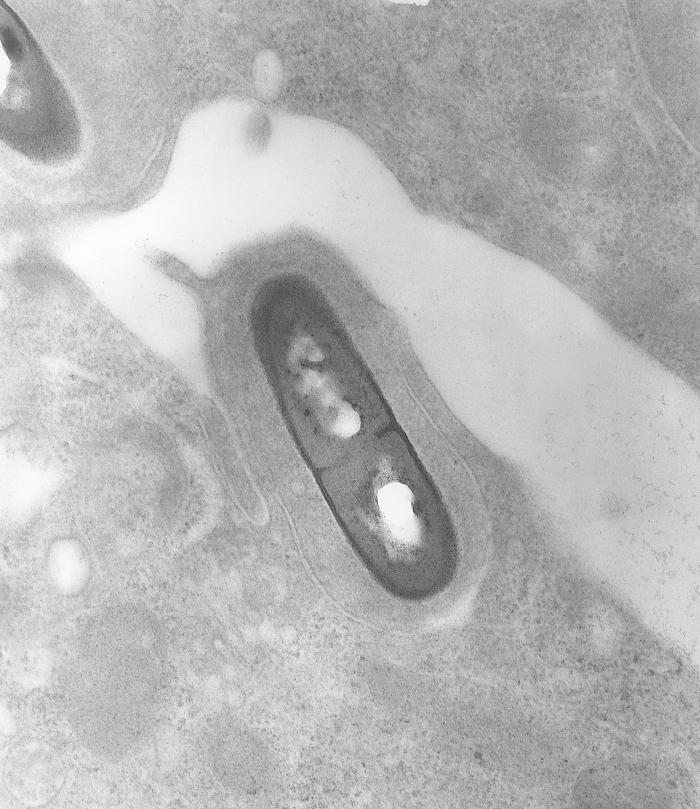
Listeria monocytogenes is a Gram-positive, facultative intracellular parasite, anaerobe, nonsporulating bacillus. Motile via flagella, L. monocytogenes can move within eukaryotic cells by explosive polymerization of actin filaments (known as comet tails or actin rockets). The name monocitogenes derives from the strong monocytic activity this organism produces in rabbits, which however, does not happen in humans.[1] Different strains of the bacteria show different pathogenic tropisms towards different tissues. It is commonly found in soil, water, vegetation and fecal material.[2]
Taxonomy
Bacteria; Firmicutes; Bacilli; Bacillales; Listeriaceae; Listeria; Listeria monocytogenes
Biology
 |
 |
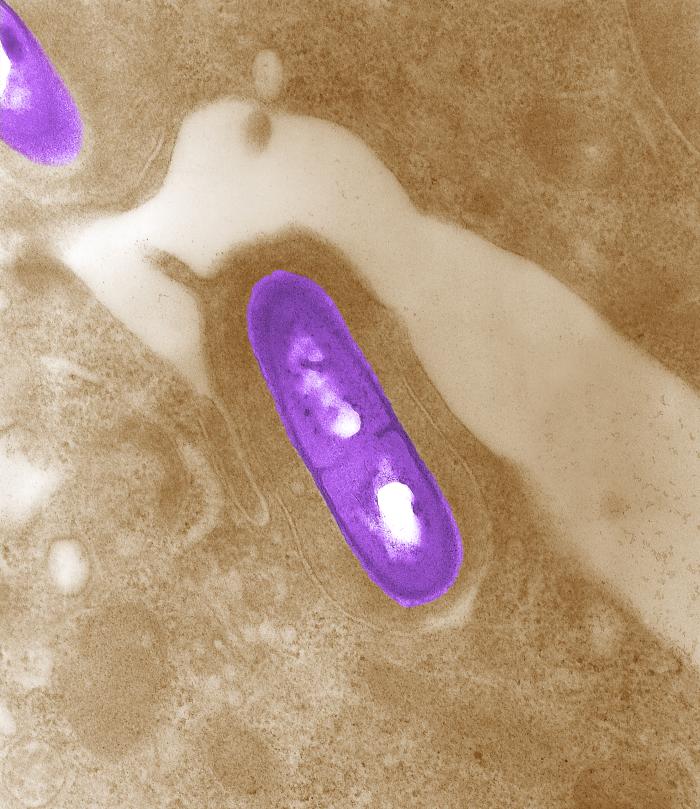 |
Listeria monocytogenes is a Gram-positive, facultative intracellular parasite, anaerobe, nonsporulating bacillus, with polar flagellae. It is a catalase-positive organism that exhibits motility, more specifically tumbling motility (between 20-25ºC). At 35 ºC the bacterium looses its motility.
Listeria produces acid and not gas in a variety of carbohydrates.[4] It has the ability to grow at temperatures between 0°C and 45ºC, which allows it to survive in a diverse array of environments such as soil, water, food products, and within the host cells. It can grown in an environment where the pH level ranges from 4.4 and 9.4.
Listeria uses the cellular machinery to move inside the host cell. It induces directed polymerization of actin by the ActA transmembrane protein, thus pushing the bacterial cell inside the host cell.
Infectious Cycle
The primary site of infection is the intestinal epithelium, where the bacteria invade non-phagocytic cells via the "zipper" mechanism:
- Uptake is stimulated by the binding of listerial internalins (Inl) to host cell adhesion factors such as E-cadherin or Met.
- This binding activates certain Rho-GTPases which subsequently bind and stabilize the Wiskott-Aldrich syndrome protein (WASp).
- WASp can then bind the Arp2/3 complex and serve as an actin nucleation point.
- Subsequent actin polymerization extends the cell membrane around the bacterium, eventually engulfing it.
- The net effect of internalin binding is to exploit the junction forming-apparatus of the host into internalizing the bacterium.
L. monocytogenes can also invade phagocytic cells (e.g. macrophages) but only requires internalins for invasion of non-phagocytic cells.
- Following internalization, the bacterium must escape from the vacuole/phagosome before fusion with a lysosome occurs. Two main virulence factors allow the bacterium to escape:
- Listeriolysin O (LLO - encoded by hly)
- Phospholipase C B (plcB).
- Secretion of LLO and PlcB disrupts the vacuolar membrane and allows the bacterium to escape into the cytoplasm where it can proliferate.
- Once in the cytoplasm, L. monocytogenes exploits host's actin filaments for the second time:
- ActA proteins associated with the old bacterial cell pole, are capable of binding the Arp2/3 complex and thus induce actin nucleation at a specific area of the bacterial cell surface (being a bacilli, L. monocytogenes septates in the middle of the cell and thus has "new pole" and another "old pole").
- Actin polymerization then propels the bacterium unidirectionally into the host cell membrane. The protrusion which is formed, may then be internalised by a neighbouring cell, forming a double-membrane vacuole from which the bacterium must escape from, using LLO and PlcB.
Tropism
Studies have shown that different strains of Listeria monocytogenes show different pathogenic tropisms towards cells of different tissues. Such is noted in humans and other animals, by the following examples:[5]
- In humans, the bacterial strain "serovar 4b" has been more frequently found in fetomaternal cases, than in those unrelated to pregnancy[6]
- In animals (sheep), two different types of listeriosis, causing meningoencephalitis and abortion, did not occur concomitantly in the same colony of bacteria[7]
Natural Reservoir
Listeria monocytogenes is broadly distributed throughout the environment. It is commonly found in soil, water, vegetation and fecal material. Animals can carry the bacterium without appearing ill and can contaminate foods of animal origin, such as meats and dairy products.[2]
L. monocytogenes has been associated with foods such as raw milk, pasteurized fluid milk[8], cheeses (particularly soft-ripened varieties), ice cream, raw vegetables, fermented raw-meat sausages, raw and cooked poultry, raw meats (of all types), and raw and smoked fish.
Its ability to grow at temperatures as low as 0°C, allows its multiplication in refrigerated foods. At refrigerated temperature such as 4°C, the amount of ferric iron in the environment promotes the growth of L. monocytogenes.[9]
Treatment
Antimicrobial regimen
- Meningitis
- Preferred regimen: Ampicillin 2g IV q4-6h ± Gentamicin 1.7 mg/kg IV q8h for more than 3 weeks
- Alternative regimen: TMP-SMX 3-5 mg/kg (trimethoprim) q6h IV for more than 3 weeks
- Bacteremia
- Preferred regimen: Ampicillin 2g IV q4-6h ± Gentamicin 1.7 mg/kg IV q8h for 2 weeks
- Alternative regimen: TMP-SMX 3-5 mg/kg (trimethoprim) q6h IV for 2 weeks
- Brain abscess or rhomboencephalitis
- Preferred regimen: Ampicillin 2g IV q4-6h ± Gentamicin 1.7 mg/kg IV q8h for 4-6 weeks
- Alternative regimen: TMP-SMX 3-5 mg/kg (trimethoprim) q6h IV for 4-6 weeks
- Gastroenteritis
References
- ↑ Mandell, Gerald L.; Bennett, John E. (John Eugene); Dolin, Raphael. (2010). Mandell, Douglas, and Bennett's principles and practice of infectious disease. Philadelphia, PA: Churchill Livingstone/Elsevier. ISBN 0-443-06839-9.
- ↑ 2.0 2.1 "Risk assessment of Listeria monocytogenes in ready-to-eat foods" (PDF).
- ↑ 3.0 3.1 3.2 "Public Health Image Library (PHIL), Centers for Disease Control and Prevention".
- ↑ Chapter 13. Non-Spore-Forming Gram-Positive Bacilli: Corynebacterium, Propionibacterium, Listeria, Erysipelothrix, Actinomycetes, & Related Pathogens ,Jawetz, Melnick, & Adelberg's Medical Microbiology, 24th Edition ,The McGraw-Hill Companies
- ↑ Vazquez-Boland, J. A.; Kuhn, M.; Berche, P.; Chakraborty, T.; Dominguez-Bernal, G.; Goebel, W.; Gonzalez-Zorn, B.; Wehland, J.; Kreft, J. (2001). "Listeria Pathogenesis and Molecular Virulence Determinants". Clinical Microbiology Reviews. 14 (3): 584–640. doi:10.1128/CMR.14.3.584-640.2001. ISSN 0893-8512.
- ↑ McLauchlin J (1990). "Distribution of serovars of Listeria monocytogenes isolated from different categories of patients with listeriosis". Eur J Clin Microbiol Infect Dis. 9 (3): 210–3. PMID 2110901.
- ↑ Low JC, Wright F, McLauchlin J, Donachie W (1993). "Serotyping and distribution of Listeria isolates from cases of ovine listeriosis". Vet Rec. 133 (7): 165–6. PMID 8236705.
- ↑ Fleming, D. W., S. L. Cochi, K. L. MacDonald, J. Brondum, P. S. Hayes, B. D. Plikaytis, M. B. Holmes, A. Audurier, C. V. Broome, and A. L. Reingold. 1985. Pasteurized milk as a vehicle of infection in an outbreak of listeriosis. N. Engl. J. Med. 312:404-407.
- ↑ Dykes, G. A., Dworaczek (Kubo), M. 2002. Influence of interactions between temperature, ferric ammonium citrate and glycine betaine on the growth of Listeria monocytogenes in a defined medium. Lett Appl Microbiol. 35(6):538-42.