Linagliptin and Metformin hydrochloride: Difference between revisions
No edit summary |
No edit summary |
||
| Line 9: | Line 9: | ||
|adverseReactions=nasopharyngitis and diarrhea | |adverseReactions=nasopharyngitis and diarrhea | ||
|blackBoxWarningTitle=<span style="color:#FF0000;">WARNING: RISK OF LACTIC ACIDOSIS</span> | |blackBoxWarningTitle=<span style="color:#FF0000;">WARNING: RISK OF LACTIC ACIDOSIS</span> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Risk | |blackBoxWarningBody=<i><span style="color:#FF0000;">Risk of lactic acidosis:</span></i> | ||
* Lactic acidosis is a rare, but serious, complication that can occur due to metformin accumulation. The risk increases with conditions such as renal impairment, sepsis, dehydration, excess alcohol intake, hepatic impairment, and acute congestive heart failure. | * Lactic acidosis is a rare, but serious, complication that can occur due to metformin accumulation. The risk increases with conditions such as renal impairment, sepsis, dehydration, excess alcohol intake, hepatic impairment, and acute congestive heart failure. | ||
| Line 46: | Line 46: | ||
*JENTADUETO has not been studied in patients with a history of pancreatitis. It is unknown whether patients with a history of pancreatitis are at an increased risk for the development of pancreatitis while using JENTADUETO. | *JENTADUETO has not been studied in patients with a history of pancreatitis. It is unknown whether patients with a history of pancreatitis are at an increased risk for the development of pancreatitis while using JENTADUETO. | ||
|offLabelAdultGuideSupport=*There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Linagliptin and Metformin hydrochloride in adult patients. | |offLabelAdultGuideSupport=*There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Linagliptin and Metformin hydrochloride in adult patients. | ||
|offLabelAdultNoGuideSupport=*There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Linagliptin and Metformin hydrochloride in adult patients. | |offLabelAdultNoGuideSupport=*There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Linagliptin and Metformin hydrochloride in adult patients. | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Linagliptin and Metformin hydrochloride in pediatric patients. | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Linagliptin and Metformin hydrochloride in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Linagliptin and Metformin hydrochloride in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Linagliptin and Metformin hydrochloride in pediatric patients. | ||
|contraindications=JENTADUETO is contraindicated in patients with: | |||
*Renal impairment (e.g., serum creatinine ≥1.5 mg/dL for men, ≥1.4 mg/dL for women, or abnormal creatinine clearance), which may also result from conditions such as cardiovascular collapse (shock), acute myocardial infarction, and septicemia. | |||
*Acute or chronic metabolic acidosis, including diabetic ketoacidosis. Diabetic ketoacidosis should be treated with insulin. | |||
*A history of hypersensitivity reaction to linagliptin, such as anaphylaxis, angioedema, exfoliative skin conditions, urticaria, or bronchial hyperreactivity. | |||
*Hypersensitivity to metformin | |||
|warnings=5.1 Lactic Acidosis | |||
Metformin | |||
Lactic acidosis is a serious, metabolic complication that can occur due to metformin accumulation during treatment with JENTADUETO and is fatal in approximately 50% of cases. Lactic acidosis may also occur in association with a number of pathophysiologic conditions, including diabetes mellitus, and whenever there is significant tissue hypoperfusion and hypoxemia. Lactic acidosis is characterized by elevated blood lactate levels (>5 mmol/L), decreased blood pH, electrolyte disturbances with an increased anion gap, and an increased lactate/pyruvate ratio. When metformin is implicated as the cause of lactic acidosis, metformin plasma levels of >5 µg/mL are generally found. | |||
The reported incidence of lactic acidosis in patients receiving metformin is approximately 0.03 cases/1000 patient-years, (with approximately 0.015 fatal cases/1000 patient-years). In more than 20,000 patient-years exposure to metformin in clinical trials, there were no reports of lactic acidosis. Reported cases have occurred primarily in diabetic patients with significant renal impairment, including both intrinsic renal disease and renal hypoperfusion, often in the setting of multiple concomitant medical/surgical problems and multiple concomitant medications. Patients with congestive heart failure requiring pharmacologic management, particularly when accompanied by hypoperfusion and hypoxemia due to unstable or acute failure, are at increased risk of lactic acidosis. The risk of lactic acidosis increases with the degree of renal impairment and the patient’s age. The risk of lactic acidosis may, therefore, be significantly decreased by regular monitoring of renal function in patients taking metformin. In particular, treatment of the elderly should be accompanied by careful monitoring of renal function. Metformin treatment should not be initiated in any patient unless measurement of creatinine clearance demonstrates that renal function is not reduced. In addition, metformin should be promptly withheld in the presence of any condition associated with hypoxemia, dehydration, or sepsis. Because impaired hepatic function may significantly limit the ability to clear lactate, metformin should be avoided in patients with clinical or laboratory evidence of hepatic impairment. Patients should be cautioned against excessive alcohol intake when taking metformin, since alcohol potentiates the effects of metformin on lactate metabolism. In addition, metformin should be temporarily discontinued prior to any intravascular radiocontrast study and for any surgical procedure necessitating restricted intake of food or fluids. Use of topiramate, a carbonic anhydrase inhibitor, in epilepsy and migraine prophylaxis may cause dose-dependent metabolic acidosis and may exacerbate the risk of metformin-induced lactic acidosis [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)]. | |||
The onset of lactic acidosis is often subtle, and accompanied by nonspecific symptoms such as malaise, myalgias, respiratory distress, increasing somnolence, and nonspecific abdominal distress. More severe acidosis may be associated with signs such as hypothermia, hypotension, and resistant bradyarrhythmias. Patients should be educated to recognize and promptly report these symptoms. If present, JENTADUETO should be discontinued until lactic acidosis is ruled out. Gastrointestinal symptoms, which are commonly reported during initiation of metformin therapy are less frequently observed in subjects on a chronic, stable, dose of metformin. Gastrointestinal symptoms in subjects on chronic, stable, dose of metformin could be caused by lactic acidosis or other serious disease. | |||
To rule out lactic acidosis, serum electrolytes, ketones, blood glucose, blood pH, lactate levels, and blood metformin levels may be useful. Levels of fasting venous plasma lactate above the upper limit of normal but less than 5 mmol/L in patients taking metformin do not necessarily indicate impending lactic acidosis and may be due to other mechanisms, such as poorly-controlled diabetes or obesity, vigorous physical activity, or technical problems in sample handling. | |||
Lactic acidosis should be suspected in any diabetic patient with metabolic acidosis lacking evidence of ketoacidosis (ketonuria and ketonemia). Lactic acidosis is a medical emergency that must be treated in a hospital setting. In a patient with lactic acidosis who is taking metformin, the drug should be discontinued immediately and supportive measures promptly instituted. Metformin is dialyzable (clearance of up to 170 mL/min under good hemodynamic conditions) and prompt hemodialysis is recommended to remove the accumulated metformin and correct the metabolic acidosis. Such management often results in prompt reversal of symptoms and recovery [see Boxed Warning]. | |||
5.2 Pancreatitis | |||
There have been postmarketing reports of acute pancreatitis, including fatal pancreatitis, in patients taking linagliptin. Take careful notice of potential signs and symptoms of pancreatitis. If pancreatitis is suspected, promptly discontinue JENTADUETO and initiate appropriate management. It is unknown whether patients with a history of pancreatitis are at increased risk for the development of pancreatitis while using JENTADUETO. | |||
5.3 Monitoring of Renal Function | |||
Although linagliptin undergoes minimal renal excretion, metformin is known to be substantially excreted by the kidney. The risk of metformin accumulation and lactic acidosis increases with the degree of renal impairment. Therefore, JENTADUETO is contraindicated in patients with renal impairment. | |||
Before initiation of therapy with JENTADUETO and at least annually thereafter, renal function should be assessed and verified to be normal. In patients in whom development of renal impairment is anticipated (e.g., elderly), renal function should be assessed more frequently and JENTADUETO discontinued if evidence of renal impairment is present. | |||
Linagliptin may be continued as a single entity tablet at the same total daily dose of 5 mg if JENTADUETO is discontinued due to evidence of renal impairment. No dose adjustment of linagliptin is recommended in patients with renal impairment. | |||
Use of concomitant medications that may affect renal function or metformin disposition: | |||
Concomitant medication(s) that may affect renal function or result in significant hemodynamic change or interfere with the disposition of metformin should be used with caution [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)]. | |||
Radiological studies and surgical procedures: | |||
Radiologic studies involving the use of intravascular iodinated contrast materials (e.g., intravenous urogram, intravenous cholangiography, angiography, and computed tomography) can lead to acute alteration of renal function and have been associated with lactic acidosis in patients receiving metformin. Therefore, in patients in whom any such study is planned, JENTADUETO should be temporarily discontinued at the time of or prior to the procedure, and withheld for 48 hours subsequent to the procedure and reinstituted only after renal function has been confirmed to be normal. | |||
JENTADUETO should be temporarily discontinued for any surgical procedure (except minor procedures not associated with restricted intake of food and fluids) and should not be restarted until the patient’s oral intake has resumed and renal function has been evaluated as normal. | |||
5.4 Impaired Hepatic Function | |||
Because impaired hepatic function has been associated with some cases of lactic acidosis with metformin therapy, JENTADUETO should generally be avoided in patients with clinical or laboratory evidence of hepatic disease [see Warnings and Precautions (5.1)]. | |||
5.5 Use with Medications Known to Cause Hypoglycemia | |||
Linagliptin | |||
Insulin secretagogues and insulin are known to cause hypoglycemia. The use of linagliptin in combination with an insulin secretagogue (e.g., sulfonylurea) was associated with a higher rate of hypoglycemia compared with placebo in a clinical trial [see Adverse Reactions (6.1)]. The use of linagliptin in combination with insulin in subjects with severe renal impairment was associated with a higher rate of hypoglycemia [see Adverse Reactions (6.1)]. Therefore, a lower dose of the insulin secretagogue or insulin may be required to reduce the risk of hypoglycemia when used in combination with JENTADUETO [see Dosage and Administration (2.2)]. | |||
Metformin | |||
Hypoglycemia does not occur in patients receiving metformin alone under usual circumstances of use, but could occur when caloric intake is deficient, when strenuous exercise is not compensated by caloric supplementation, or during concomitant use with other glucose-lowering agents (such as SUs and insulin) or ethanol. Elderly, debilitated, or malnourished patients, and those with adrenal or pituitary insufficiency or alcohol intoxication are particularly susceptible to hypoglycemic effects. Hypoglycemia may be difficult to recognize in the elderly, and in people who are taking β-adrenergic blocking drugs. | |||
5.6 Hypersensitivity Reactions | |||
There have been postmarketing reports of serious hypersensitivity reactions in patients treated with linagliptin (one of the components of JENTADUETO). These reactions include anaphylaxis, angioedema, and exfoliative skin conditions. Onset of these reactions occurred within the first 3 months after initiation of treatment with linagliptin, with some reports occurring after the first dose. If a serious hypersensitivity reaction is suspected, discontinue JENTADUETO, assess for other potential causes for the event, and institute alternative treatment for diabetes. | |||
Angioedema has also been reported with other dipeptidyl peptidase-4 (DPP-4) inhibitors. Use caution in a patient with a history of angioedema to another DPP-4 inhibitor because it is unknown whether such patients will be predisposed to angioedema with JENTADUETO. | |||
5.7 Vitamin B12 Levels | |||
In controlled, 29-week clinical trials of metformin, a decrease to subnormal levels of previously normal serum vitamin B12 levels, without clinical manifestations, was observed in approximately 7% of metformin-treated patients. Such decrease, possibly due to interference with B12 absorption from the B12-intrinsic factor complex, is, however, very rarely associated with anemia or neurologic manifestations due to the short duration (<1 year) of the clinical trials. This risk may be more relevant to patients receiving long-term treatment with metformin, and adverse hematologic and neurologic reactions have been reported postmarketing. The decrease in vitamin B12 levels appears to be rapidly reversible with discontinuation of metformin or vitamin B12 supplementation. Measurement of hematologic parameters on an annual basis is advised in patients on JENTADUETO and any apparent abnormalities should be appropriately investigated and managed. Certain individuals (those with inadequate vitamin B12 or calcium intake or absorption) appear to be predisposed to developing subnormal vitamin B12 levels. In these patients, routine serum vitamin B12 measurement at 2- to 3-year intervals may be useful. | |||
5.8 Alcohol Intake | |||
Alcohol is known to potentiate the effect of metformin on lactate metabolism. Patients, therefore, should be warned against excessive alcohol intake while receiving JENTADUETO [see Warnings and Precautions (5.1)]. | |||
5.9 Hypoxic States | |||
Cardiovascular collapse (shock) from whatever cause (e.g., acute congestive heart failure, acute myocardial infarction, and other conditions characterized by hypoxemia) have been associated with lactic acidosis and may also cause prerenal azotemia. When such events occur in patients on JENTADUETO therapy, the drug should be promptly discontinued [see Warnings and Precautions (5.1)]. | |||
5.10 Macrovascular Outcomes | |||
There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with linagliptin or metformin or any other antidiabetic drug. | |||
|clinicalTrials=6.1 Clinical Trials Experience | |||
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | |||
Linagliptin/Metformin | |||
The safety of concomitantly administered linagliptin (daily dose 5 mg) and metformin (mean daily dose of approximately 1800 mg) has been evaluated in 2816 patients with type 2 diabetes mellitus treated for ≥12 weeks in clinical trials. | |||
Three placebo-controlled studies with linagliptin + metformin were conducted: 2 studies were 24 weeks in duration, 1 study was 12 weeks in duration. In the 3 placebo-controlled clinical studies, adverse events which occurred in ≥5% of patients receiving linagliptin + metformin (n=875) and were more common than in patients given placebo + metformin (n=539) included nasopharyngitis (5.7% vs 4.3%). | |||
In a 24-week factorial design study, adverse events reported in ≥5% of patients receiving linagliptin + metformin and were more common than in patients given placebo are shown in Table 1. | |||
[[File:Linagliptin and metformin table 1.png|600px|thumbnail|left]] | |||
{{clear}} | |||
Other adverse reactions reported in clinical studies with treatment of linagliptin + metformin were hypersensitivity (e.g., urticaria, angioedema, or bronchial hyperreactivity), cough, decreased appetite, nausea, vomiting, pruritus, and pancreatitis. | |||
Linagliptin | |||
Adverse reactions reported in ≥2% of patients treated with linagliptin 5 mg and more commonly than in patients treated with placebo included: nasopharyngitis (7.0% vs 6.1%), diarrhea (3.3% vs 3.0%), and cough (2.1% vs 1.4%). | |||
Rates for other adverse reactions for linagliptin 5 mg vs placebo when linagliptin was used in combination with specific anti-diabetic agents were: urinary tract infection (3.1% vs 0%) and hypertriglyceridemia (2.4% vs 0%) when linagliptin was used as add-on to sulfonylurea; hyperlipidemia (2.7% vs 0.8%) and weight increased (2.3% vs 0.8%) when linagliptin was used as add-on to pioglitazone; and constipation (2.1% vs 1%) when linagliptin was used as add-on to basal insulin therapy. | |||
Other adverse reactions reported in clinical studies with treatment of linagliptin monotherapy were hypersensitivity (e.g., urticaria, angioedema, localized skin exfoliation, or bronchial hyperreactivity) and myalgia. In the clinical trial program, pancreatitis was reported in 15.2 cases per 10,000 patient year exposure while being treated with linagliptin compared with 3.7 cases per 10,000 patient year exposure while being treated with comparator (placebo and active comparator, sulfonylurea). Three additional cases of pancreatitis were reported following the last administered dose of linagliptin. | |||
Metformin | |||
The most common adverse reactions due to initiation of metformin are diarrhea, nausea/vomiting, flatulence, asthenia, indigestion, abdominal discomfort, and headache. | |||
Long-term treatment with metformin has been associated with a decrease in vitamin B12 absorption which may very rarely result in clinically significant vitamin B12 deficiency (e.g., megaloblastic anemia) [see Warnings and Precautions (5.5)]. | |||
Hypoglycemia | |||
Linagliptin/Metformin | |||
In a 24-week factorial design study, hypoglycemia was reported in 4 (1.4%) of 286 subjects treated with linagliptin + metformin, 6 (2.1%) of 291 subjects treated with metformin, and 1 (1.4%) of 72 subjects treated with placebo. When linagliptin was administered in combination with metformin and a sulfonylurea, 181 (22.9%) of 792 patients reported hypoglycemia compared with 39 (14.8%) of 263 patients administered placebo in combination with metformin and sulfonylurea. Adverse reactions of hypoglycemia were based on all reports of hypoglycemia. A concurrent glucose measurement was not required or was normal in some patients. Therefore, it is not possible to conclusively determine that all these reports reflect true hypoglycemia. | |||
Linagliptin | |||
In the study of patients receiving linagliptin as add-on therapy to a stable dose of insulin for up to 52 weeks (n=1261), no significant difference in the incidence of investigator reported hypoglycemia, defined as all symptomatic or asymptomatic episodes with a self-measured blood glucose ≤70 mg/dL, was noted between the linagliptin- (31.4%) and placebo- (32.9%) treated groups. | |||
Use in Renal Impairment | |||
Linagliptin was compared to placebo as add-on to pre-existing antidiabetic therapy over 52 weeks in 133 patients with severe renal impairment (estimated GFR <30 mL/min). For the initial 12 weeks of the study, background antidiabetic therapy was kept stable and included insulin, sulfonylurea, glinides, and pioglitazone. For the remainder of the trial, dose adjustments in antidiabetic background therapy were allowed. | |||
In general, the incidence of adverse events including severe hypoglycemia was similar to those reported in other linagliptin trials. The observed incidence of hypoglycemia was higher (linagliptin, 63% compared to placebo, 49%) due to an increase in asymptomatic hypoglycemic events especially during the first 12 weeks when background glycemic therapies were kept stable. Ten linagliptin-treated patients (15%) and 11 placebo-treated patients (17%) reported at least one episode of confirmed symptomatic hypoglycemia (accompanying finger stick glucose ≤54 mg/dL). During the same time period, severe hypoglycemic events, defined as an event requiring the assistance of another person to actively administer carbohydrate, glucagon or other resuscitative actions, were reported in 3 (4.4%) linagliptin-treated patients and 3 (4.6%) placebo-treated patients. Events that were considered life-threatening or required hospitalization were reported in 2 (2.9%) patients on linagliptin and 1 (1.5%) patient on placebo. | |||
Renal function as measured by mean eGFR and creatinine clearance did not change over 52 weeks’ treatment compared to placebo. | |||
Laboratory Tests | |||
Changes in laboratory findings were similar in patients treated with linagliptin + metformin compared to patients treated with placebo + metformin. Changes in laboratory values that occurred more frequently in the linagliptin + metformin group and ≥1% more than in the placebo group were not detected. | |||
No clinically meaningful changes in vital signs were observed in patients treated with linagliptin. | |||
[[File:Linagliptin and metformin table 2.png|thumbnail]] | |||
|postmarketing=*The following adverse reactions have been identified during postapproval use of linagliptin. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure. | |||
:*Acute pancreatitis, including fatal pancreatitis | |||
:*Hypersensitivity reactions including anaphylaxis, angioedema, and exfoliative skin conditions | |||
:*Rash | |||
|drugInteractions=7.1 Drug Interactions with Metformin | |||
Cationic Drugs | |||
Cationic drugs (e.g., amiloride, digoxin, morphine, procainamide, quinidine, quinine, ranitidine, triamterene, trimethoprim, or vancomycin) that are eliminated by renal tubular secretion theoretically have the potential for interaction with metformin by competing for common renal tubular transport systems. Although such interactions remain theoretical (except for cimetidine), careful patient monitoring and dose adjustment of JENTADUETO and/or the interfering drug is recommended in patients who are taking cationic medications that are excreted via the proximal renal tubular secretory system [see Warnings and Precautions (5.3) and Clinical Pharmacology (12.3)]. | |||
Carbonic Anhydrase Inhibitors | |||
Topiramate or other carbonic anhydrase inhibitors (e.g., zonisamide, acetazolamide or dichlorphenamide) frequently decrease serum bicarbonate and induce non-anion gap, hyperchloremic metabolic acidosis. Concomitant use of these drugs may induce metabolic acidosis. Use these drugs with caution in patients treated with JENTADUETO, as the risk of lactic acidosis may increase [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)]. | |||
7.2 Drug Interactions with Linagliptin | |||
Inducers of P-glycoprotein and CYP3A4 Enzymes | |||
Rifampin decreased linagliptin exposure, suggesting that the efficacy of linagliptin may be reduced when administered in combination with a strong P-gp inducer or CYP 3A4 inducer. As JENTADUETO is a fixed-dose combination of linagliptin and metformin, use of alternative treatments (not containing linagliptin) is strongly recommended when concomitant treatment with a strong P-gp or CYP 3A4 inducer is necessary [see Clinical Pharmacology (12.3)]. | |||
7.3 Drugs Affecting Glycemic Control | |||
Certain drugs tend to produce hyperglycemia and may lead to loss of glycemic control. These drugs include the thiazides and other diuretics, corticosteroids, phenothiazines, thyroid products, estrogens, oral contraceptives, phenytoin, nicotinic acid, sympathomimetics, calcium channel blocking drugs, and isoniazid. When such drugs are administered to a patient receiving JENTADUETO, the patient should be closely observed to maintain adequate glycemic control [see Clinical Pharmacology (12.3)]. When such drugs are withdrawn from a patient receiving JENTADUETO, the patient should be observed closely for hypoglycemia. | |||
|FDAPregCat=B | |||
|useInPregnancyFDA=Pregnancy Category B | |||
JENTADUETO | |||
There are no adequate and well controlled studies in pregnant women with JENTADUETO or its individual components, and some clinical data is available for metformin which indicate that the risk for major malformations was not increased when metformin is taken during the first trimester in pregnancy. In addition, metformin was not associated with increased perinatal complications. Nevertheless, because these clinical data cannot rule out the possibility of harm, JENTADUETO should be used during pregnancy only if clearly needed. | |||
JENTADUETO was not teratogenic when administered to Wistar Han rats during the period of organogenesis at doses similar to clinical exposure. At higher maternally toxic doses (9 and 23 times the clinical dose based on exposure), the metformin component of the combination was associated with an increased incidence of fetal rib and scapula malformations. | |||
Linagliptin | |||
Linagliptin was not teratogenic when administered to pregnant Wistar Han rats and Himalayan rabbits during the period of organogenesis at doses up to 240 mg/kg and 150 mg/kg, respectively. These doses represent approximately 943 times the clinical dose in rats and 1943 times the clinical dose in rabbits, based on exposure. No functional, behavioral, or reproductive toxicity was observed in offspring of female Wistar Han rats when administered linagliptin from gestation day 6 to lactation day 21 at a dose 49 times the maximum recommended human dose, based on exposure. | |||
Linagliptin crosses the placenta into the fetus following oral dosing in pregnant rats and rabbits. | |||
Metformin Hydrochloride | |||
Metformin has been studied for embryo-fetal effects in 2 rat strains and in rabbits. Metformin was not teratogenic in Sprague Dawley rats up to 600 mg/kg or in Wistar Han rats up to 200 mg/kg (2-3 times the clinical dose based on body surface area or exposure, respectively). At higher maternally toxic doses (9 and 23 times the clinical dose based on exposure), an increased incidence of rib and scapula skeletal malformations was observed in the Wistar Han strain. Metformin was not teratogenic in rabbits at doses up to 140 mg/kg (similar to clinical dose based on body surface area). | |||
Metformin administered to female Sprague Dawley rats from gestation day 6 to lactation day 21 up to 600 mg/kg/day (2 times the maximum clinical dose based on body surface area) had no effect on prenatal or postnatal development of offspring. | |||
Metformin crosses the placenta into the fetus in rats and humans. | |||
|useInNursing=No studies in lactating animals have been conducted with the combined components of JENTADUETO. In studies performed with the individual components, both linagliptin and metformin were secreted in the milk of lactating rats. It is not known whether linagliptin is excreted in human milk. Metformin is excreted in human milk in low concentrations. Because the potential for hypoglycemia in nursing infants may exist, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. | |||
|useInPed=Safety and effectiveness of JENTADUETO in pediatric patients under 18 years of age have not been established. | |||
|useInGeri=Linagliptin is minimally excreted by the kidney; however, metformin is substantially excreted by the kidney. Considering that aging can be associated with reduced renal function, JENTADUETO should be used with caution as age increases [see Warnings and Precautions (5.1, 5.3) and Clinical Pharmacology (12.3)]. | |||
Linagliptin | |||
There were 4040 type 2 diabetes patients treated with linagliptin 5 mg from 15 clinical trials of linagliptin; 1085 (27%) patients were 65 years and over, while 131 (3%) were 75 years and over. Of these patients, 2566 were enrolled in 12 double-blind placebo-controlled studies; 591 (23%) were 65 years and over, while 82 (3%) were 75 years and over. No overall differences in safety or effectiveness were observed between patients 65 years and over and younger patients. Therefore, no dose adjustment is recommended in the elderly population. While clinical studies of linagliptin have not identified differences in response between the elderly and younger patients, greater sensitivity of some older individuals cannot be ruled out. | |||
Metformin | |||
Controlled clinical studies of metformin did not include sufficient numbers of elderly patients to determine whether they respond differently from younger patients, although other reported clinical experience has not identified differences in responses between the elderly and young patients. The initial and maintenance dosing of metformin should be conservative in patients with advanced age, due to the potential for decreased renal function in this population. Any dose adjustment should be based on a careful assessment of renal function [see Contraindications (4), Warnings and Precautions (5.3), and Clinical Pharmacology (12.3)]. | |||
|administration='''Recommended Dosing''' | |||
*The dosage of JENTADUETO should be individualized on the basis of both effectiveness and tolerability, while not exceeding the maximum recommended dose of 2.5 mg linagliptin/1000 mg metformin hydrochloride twice daily. JENTADUETO should be given twice daily with meals. Dose escalation should be gradual to reduce the gastrointestinal (GI) side effects associated with metformin use. For available dosage forms and strengths see. | |||
*Recommended starting dose: | |||
:*In patients currently not treated with metformin, initiate treatment with 2.5 mg linagliptin/500 mg metformin hydrochloride twice daily | |||
:*In patients already treated with metformin, start with 2.5 mg linagliptin and the current dose of metformin taken at each of the two daily meals (e.g., a patient on metformin 1000 mg twice daily would be started on 2.5 mg linagliptin/1000 mg metformin hydrochloride twice daily with meals). | |||
:*Patients already treated with linagliptin and metformin individual components may be switched to JENTADUETO containing the same doses of each component. | |||
*No studies have been performed specifically examining the safety and efficacy of JENTADUETO in patients previously treated with other oral antihyperglycemic agents and switched to JENTADUETO. Any change in therapy of type 2 diabetes mellitus should be undertaken with care and appropriate monitoring as changes in glycemic control can occur. | |||
'''Concomitant Use with an Insulin Secretagogue (e.g., Sulfonylurea) or with Insulin''' | |||
*When JENTADUETO is used in combination with an insulin secretagogue (e.g., sulfonylurea) or with insulin, a lower dose of the insulin secretagogue or insulin may be required to reduce the risk of hypoglycemia. | |||
====Dosage forms and strengths==== | |||
*JENTADUETO is a combination of linagliptin and metformin hydrochloride. JENTADUETO tablets are available in the following dosage forms and strengths: | |||
:*2.5 mg linagliptin/500 mg metformin hydrochloride tablets are light yellow, oval, biconvex tablets debossed with “D2/500” on one side and the Boehringer Ingelheim logo on the other side | |||
:*2.5 mg linagliptin/850 mg metformin hydrochloride tablets are light orange, oval, biconvex tablets debossed with “D2/850” on one side and the Boehringer Ingelheim logo on the other side | |||
:*2.5 mg linagliptin/1000 mg metformin hydrochloride tablets are light pink, oval, biconvex tablets debossed with “D2/1000” on one side and the Boehringer Ingelheim logo on the other side | |||
|overdose=*In the event of an overdose with JENTADUETO, contact the Poison Control Center. Employ the usual supportive measures (e.g., remove unabsorbed material from the gastrointestinal tract, employ clinical monitoring, and institute supportive treatment) as dictated by the patient’s clinical status. Removal of linagliptin by hemodialysis or peritoneal dialysis is unlikely. However, metformin is dialyzable with a clearance of up to 170 mL/min under good hemodynamic conditions. Therefore, hemodialysis may be useful partly for removal of accumulated metformin from patients in whom JENTADUETO overdosage is suspected. | |||
Linagliptin | |||
During controlled clinical trials in healthy subjects, with single doses of up to 600 mg of linagliptin (equivalent to 120 times the recommended daily dose), there were no dose-related clinical adverse drug reactions. There is no experience with doses above 600 mg in humans. | |||
Metformin | |||
Overdose of metformin has occurred, including ingestion of amounts greater than 50 grams. Hypoglycemia was reported in approximately 10% of cases, but no causal association with metformin has been established. Lactic acidosis has been reported in approximately 32% of metformin overdose cases [see Boxed Warning and Warnings and Precautions (5.1)]. | |||
|mechAction=JENTADUETO | |||
JENTADUETO combines 2 antihyperglycemic agents with complementary mechanisms of action to improve glycemic control in patients with type 2 diabetes mellitus: linagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor, and metformin, a member of the biguanide class. | |||
Linagliptin | |||
Linagliptin is an inhibitor of DPP-4, an enzyme that degrades the incretin hormones glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). Thus, linagliptin increases the concentrations of active incretin hormones, stimulating the release of insulin in a glucose-dependent manner and decreasing the levels of glucagon in the circulation. Both incretin hormones are involved in the physiological regulation of glucose homeostasis. Incretin hormones are secreted at a low basal level throughout the day and levels rise immediately after meal intake. GLP-1 and GIP increase insulin biosynthesis and secretion from pancreatic beta cells in the presence of normal and elevated blood glucose levels. Furthermore, GLP-1 also reduces glucagon secretion from pancreatic alpha cells, resulting in a reduction in hepatic glucose output. | |||
Metformin | |||
Metformin is an antihyperglycemic agent which improves glucose tolerance in patients with type 2 diabetes mellitus, lowering both basal and postprandial plasma glucose. Its pharmacologic mechanisms of action are different from other classes of oral antihyperglycemic agents. Metformin decreases hepatic glucose production, decreases intestinal absorption of glucose, and improves insulin sensitivity by increasing peripheral glucose uptake and utilization. Unlike SUs, metformin does not produce hypoglycemia in either patients with type 2 diabetes mellitus or normal subjects (except in special circumstances) [see Warnings and Precautions (5.10)] and does not cause hyperinsulinemia. With metformin therapy, insulin secretion remains unchanged while fasting insulin levels and day-long plasma insulin response may actually decrease. | |||
|structure=JENTADUETO tablets contain 2 oral antihyperglycemic drugs used in the management of type 2 diabetes mellitus: linagliptin and metformin hydrochloride. | |||
Linagliptin | |||
Linagliptin is an orally-active inhibitor of the dipeptidyl peptidase-4 (DPP-4) enzyme. | |||
Linagliptin is described chemically as 1H-Purine-2,6-dione, 8-[(3R)-3-amino-1-piperidinyl]-7-(2-butyn-1-yl)-3,7-dihydro-3-methyl-1-[(4-methyl-2-quinazolinyl)methyl]- | |||
The empirical formula is C25H28N8O2 and the molecular weight is 472.54 g/mol. The structural formula is: | |||
Linagliptin structure | |||
Linagliptin is a white to yellowish, not or only slightly hygroscopic solid substance. It is very slightly soluble in water (0.9 mg/mL). Linagliptin is soluble in methanol (ca. 60 mg/mL), sparingly soluble in ethanol (ca. 10 mg/mL), very slightly soluble in isopropanol (<1 mg/mL), and very slightly soluble in acetone (ca. 1 mg/mL). | |||
Metformin Hydrochloride | |||
Metformin hydrochloride (N,N-dimethylimidodicarbonimidic diamide hydrochloride) is not chemically or pharmacologically related to any other classes of oral antihyperglycemic agents. Metformin hydrochloride is a white to off-white crystalline compound with a molecular formula of C4H11N5•HCl and a molecular weight of 165.63. Metformin hydrochloride is freely soluble in water and is practically insoluble in acetone, ether, and chloroform. The pKa of metformin is 12.4. The pH of a 1% aqueous solution of metformin hydrochloride is 6.68. The structural formula is: | |||
Metformin structure | |||
JENTADUETO | |||
JENTADUETO is available for oral administration as tablets containing 2.5 mg linagliptin and 500 mg metformin hydrochloride (JENTADUETO 2.5 mg/500 mg), 850 mg metformin hydrochloride (JENTADUETO 2.5 mg/850 mg) or 1000 mg metformin hydrochloride (JENTADUETO 2.5 mg/1000 mg). Each film-coated tablet of JENTADUETO contains the following inactive ingredients: arginine, corn starch, copovidone, colloidal silicon dioxide, magnesium stearate, titanium dioxide, propylene glycol, hypromellose, talc, yellow ferric oxide (2.5 mg/500 mg; 2.5 mg/850 mg) and/or red ferric oxide (2.5 mg/850 mg; 2.5 mg/1000 mg). | |||
|PD=Linagliptin | |||
Linagliptin binds to DPP-4 in a reversible manner and increases the concentrations of incretin hormones. Linagliptin glucose-dependently increases insulin secretion and lowers glucagon secretion, thus resulting in a better regulation of the glucose homeostasis. Linagliptin binds selectively to DPP-4 and selectively inhibits DPP-4, but not DPP-8 or DPP-9 activity in vitro at concentrations approximating therapeutic exposures. | |||
Cardiac Electrophysiology | |||
In a randomized, placebo-controlled, active-comparator, 4-way crossover study, 36 healthy subjects were administered a single oral dose of linagliptin 5 mg, linagliptin 100 mg (20 times the recommended dose), moxifloxacin, and placebo. No increase in QTc was observed with either the recommended dose of 5 mg or the 100-mg dose. At the 100-mg dose, peak linagliptin plasma concentrations were approximately 38-fold higher than the peak concentrations following a 5-mg dose. | |||
|PK=JENTADUETO | |||
The results of a bioequivalence study in healthy subjects demonstrated that JENTADUETO (linagliptin/metformin hydrochloride) 2.5 mg/500 mg, 2.5 mg/850 mg, and 2.5 mg/1000 mg combination tablets are bioequivalent to coadministration of corresponding doses of linagliptin and metformin as individual tablets. Administration of linagliptin 2.5 mg/metformin hydrochloride 1000 mg fixed-dose combination with food resulted in no change in overall exposure of linagliptin. There was no change in metformin AUC; however, mean peak serum concentration of metformin was decreased by 18% when administered with food. A delayed time-to-peak serum concentrations by 2 hours was observed for metformin under fed conditions. These changes are not likely to be clinically significant. | |||
Absorption | |||
Linagliptin | |||
The absolute bioavailability of linagliptin is approximately 30%. Following oral administration, plasma concentrations of linagliptin decline in at least a biphasic manner with a long terminal half-life (>100 hours), related to the saturable binding of linagliptin to DPP-4. However, the prolonged elimination does not contribute to the accumulation of the drug. The effective half-life for accumulation of linagliptin, as determined from oral administration of multiple doses of linagliptin 5 mg, is approximately 12 hours. After once-daily dosing, steady state plasma concentrations of linagliptin 5 mg are reached by the third dose, and Cmax and AUC increased by a factor of 1.3 at steady-state compared with the first dose. Plasma AUC of linagliptin increased in a less than dose-proportional manner in the dose range of 1 to 10 mg. The pharmacokinetics of linagliptin is similar in healthy subjects and in patients with type 2 diabetes. | |||
Metformin | |||
The absolute bioavailability of a metformin hydrochloride 500-mg tablet given under fasting conditions is approximately 50% to 60%. Studies using single oral doses of metformin tablets 500 mg to 1500 mg, and 850 mg to 2550 mg, indicate that there is a lack of dose proportionality with increasing doses, which is due to decreased absorption rather than an alteration in elimination. | |||
Distribution | |||
Linagliptin | |||
The mean apparent volume of distribution at steady state following a single intravenous dose of linagliptin 5 mg to healthy subjects is approximately 1110 L, indicating that linagliptin extensively distributes to the tissues. Plasma protein binding of linagliptin is concentration-dependent decreasing from about 99% at 1 nmol/L to 75% to 89% at ≥30 nmol/L, reflecting saturation of binding to DPP-4 with increasing concentration of linagliptin. At high concentrations, where DPP-4 is fully saturated, 70% to 80% of linagliptin remains bound to plasma proteins and 20% to 30% is unbound in plasma. Plasma binding is not altered in patients with renal or hepatic impairment. | |||
Metformin | |||
The apparent volume of distribution (V/F) of metformin following single oral doses of immediate-release metformin hydrochloride tablets 850 mg averaged 654±358 L. Metformin is negligibly bound to plasma proteins, in contrast to SUs, which are more than 90% protein bound. Metformin partitions into erythrocytes, most likely as a function of time. At usual clinical doses and dosing schedules of metformin tablets, steady-state plasma concentrations of metformin are reached within 24 to 48 hours and are generally <1 mcg/mL. During controlled clinical trials of metformin, maximum metformin plasma levels did not exceed 5 mcg/mL, even at maximum doses. | |||
Metabolism | |||
Linagliptin | |||
Following oral administration, the majority (about 90%) of linagliptin is excreted unchanged, indicating that metabolism represents a minor elimination pathway. A small fraction of absorbed linagliptin is metabolized to a pharmacologically inactive metabolite, which shows a steady-state exposure of 13.3% relative to linagliptin. | |||
Metformin | |||
Intravenous single-dose studies in normal subjects demonstrate that metformin is excreted unchanged in the urine and does not undergo hepatic metabolism (no metabolites have been identified in humans) nor biliary excretion. | |||
Excretion | |||
Linagliptin | |||
Following administration of an oral [14C]linagliptin dose to healthy subjects, approximately 85% of the administered radioactivity was eliminated via the enterohepatic system (80%) or urine (5%) within 4 days of dosing. Renal clearance at steady state was approximately 70 mL/min. | |||
Metformin | |||
Renal clearance is approximately 3.5 times greater than creatinine clearance, which indicates that tubular secretion is the major route of metformin elimination. Following oral administration, approximately 90% of the absorbed drug is eliminated via the renal route within the first 24 hours, with a plasma elimination half-life of approximately 6.2 hours. In blood, the elimination half-life is approximately 17.6 hours, suggesting that the erythrocyte mass may be a compartment of distribution. | |||
Specific Populations | |||
Renal Impairment | |||
JENTADUETO: Studies characterizing the pharmacokinetics of linagliptin and metformin after administration of JENTADUETO in renally impaired patients have not been performed. Since metformin is contraindicated in patients with renal impairment, use of JENTADUETO is also contraindicated in patients with renal impairment (e.g., serum creatinine ≥1.5 mg/dL [males] or ≥1.4 mg/dL [females], or abnormal creatinine clearance) [see Contraindications (4) and Warnings and Precautions (5.3)]. | |||
Linagliptin: Under steady-state conditions, linagliptin exposure in patients with mild renal impairment was comparable to healthy subjects. In patients with moderate renal impairment under steady-state conditions, mean exposure of linagliptin increased (AUCτ,ss by 71% and Cmax by 46%) compared with healthy subjects. This increase was not associated with a prolonged accumulation half-life, terminal half-life, or an increased accumulation factor. Renal excretion of linagliptin was below 5% of the administered dose and was not affected by decreased renal function. | |||
Patients with type 2 diabetes mellitus and severe renal impairment showed steady-state exposure approximately 40% higher than that of patients with type 2 diabetes mellitus and normal renal function (increase in AUC by 42% and Cmax by 35%). For both type 2 diabetes mellitus groups, renal excretion was below 7% of the administered dose. | |||
Metformin: In patients with decreased renal function (based on measured creatinine clearance), the plasma and blood half-life of metformin is prolonged and the renal clearance is decreased in proportion to the decrease in creatinine clearance [see Contraindications (4) and Warnings and Precautions (5.3)]. | |||
Hepatic Impairment | |||
JENTADUETO: Studies characterizing the pharmacokinetics of linagliptin and metformin after administration of JENTADUETO in hepatically impaired patients have not been performed. However, use of metformin alone in patients with hepatic impairment has been associated with some cases of lactic acidosis. Therefore, use of JENTADUETO is not recommended in patients with hepatic impairment [see Warnings and Precautions (5.4)]. | |||
Linagliptin: In patients with mild hepatic impairment (Child-Pugh class A) steady-state exposure (AUCτ,ss) of linagliptin was approximately 25% lower and Cmax,ss was approximately 36% lower than in healthy subjects. In patients with moderate hepatic impairment (Child-Pugh class B), AUCss of linagliptin was about 14% lower and Cmax,ss was approximately 8% lower than in healthy subjects. Patients with severe hepatic impairment (Child-Pugh class C) had comparable exposure of linagliptin in terms of AUC0-24 and approximately 23% lower Cmax compared with healthy subjects. Reductions in the pharmacokinetic parameters seen in patients with hepatic impairment did not result in reductions in DPP-4 inhibition. | |||
Metformin hydrochloride: No pharmacokinetic studies of metformin have been conducted in patients with hepatic impairment. | |||
Body Mass Index (BMI)/Weight | |||
Linagliptin: BMI/Weight had no clinically meaningful effect on the pharmacokinetics of linagliptin based on a population pharmacokinetic analysis. | |||
Gender | |||
Linagliptin: Gender had no clinically meaningful effect on the pharmacokinetics of linagliptin based on a population pharmacokinetic analysis. | |||
Metformin hydrochloride: Metformin pharmacokinetic parameters did not differ significantly between normal subjects and patients with type 2 diabetes mellitus when analyzed according to gender. Similarly, in controlled clinical studies in patients with type 2 diabetes mellitus, the antihyperglycemic effect of metformin was comparable in males and females. | |||
Geriatric | |||
JENTADUETO: Studies characterizing the pharmacokinetics of linagliptin and metformin after administration of JENTADUETO in geriatric patients have not been performed. Based on the metformin component, JENTADUETO treatment should not be initiated in patients ≥80 years of age unless measurement of creatinine clearance demonstrates that renal function is not reduced [see Warnings and Precautions (5.1, 5.3) and Use in Specific Populations (8.5)]. | |||
Linagliptin: Age did not have a clinically meaningful impact on the pharmacokinetics of linagliptin based on a population pharmacokinetic analysis. | |||
Metformin hydrochloride: Limited data from controlled pharmacokinetic studies of metformin in healthy elderly subjects suggest that total plasma clearance of metformin is decreased, the half-life is prolonged, and Cmax is increased, compared with healthy young subjects. From these data, it appears that the change in metformin pharmacokinetics with aging is primarily accounted for by a change in renal function. | |||
Pediatric | |||
Studies characterizing the pharmacokinetics of linagliptin and metformin after administration of JENTADUETO in pediatric patients have not yet been performed. | |||
Race | |||
Linagliptin: Race had no clinically meaningful effect on the pharmacokinetics of linagliptin based on available pharmacokinetic data, including subjects of White, Hispanic, Black, and Asian racial groups. | |||
Metformin hydrochloride: No studies of metformin pharmacokinetic parameters according to race have been performed. In controlled clinical studies of metformin in patients with type 2 diabetes mellitus, the antihyperglycemic effect was comparable in Caucasians (n=249), Blacks (n=51), and Hispanics (n=24). | |||
Drug Interactions | |||
Pharmacokinetic drug interaction studies with JENTADUETO have not been performed; however, such studies have been conducted with the individual components of JENTADUETO (linagliptin and metformin hydrochloride). | |||
Linagliptin | |||
In vitro Assessment of Drug Interactions | |||
Linagliptin is a weak to moderate inhibitor of CYP isozyme CYP3A4, but does not inhibit other CYP isozymes and is not an inducer of CYP isozymes, including CYP1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, and 4A11. | |||
Linagliptin is a P-glycoprotein (P-gp) substrate, and inhibits P-gp mediated transport of digoxin at high concentrations. Based on these results and in vivo drug interaction studies, linagliptin is considered unlikely to cause interactions with other P-gp substrates at therapeutic concentrations. | |||
In vivo Assessment of Drug Interactions | |||
Strong inducers of CYP3A4 or P-gp (e.g., rifampin) decrease exposure to linagliptin to subtherapeutic and likely ineffective concentrations. For patients requiring use of such drugs, an alternative to linagliptin is strongly recommended. In vivo studies indicated evidence of a low propensity for causing drug interactions with substrates of CYP3A4, CYP2C9, CYP2C8, P-gp, and OCT. No dose adjustment of linagliptin is recommended based on results of the described pharmacokinetic studies. | |||
|alcohol=Alcohol-Linagliptin and Metformin hydrochloride interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Linagliptin and Metformin hydrochloride interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | }} | ||
Revision as of 20:28, 9 October 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Deepika Beereddy, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: RISK OF LACTIC ACIDOSIS
See full prescribing information for complete Boxed Warning.
Risk of lactic acidosis:
|
Overview
Linagliptin and Metformin hydrochloride is a hypoglycemic agent that is FDA approved for the treatment of type 2 diabetes mellitus. There is a Black Box Warning for this drug as shown here. Common adverse reactions include nasopharyngitis and diarrhea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
TYpe 2 Diabetes Mellitus
- JENTADUETO tablets are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus when treatment with both linagliptin and metformin is appropriate.
Important Limitations of Use
- JENTADUETO should not be used in patients with type 1 diabetes or for the treatment of diabetic ketoacidosis, as it would not be effective in these settings.
- Dosing information:
- Recommended Dosing:
- The dosage of JENTADUETO should be individualized on the basis of both effectiveness and tolerability, while not exceeding the maximum recommended dose of 2.5 mg linagliptin/1000 mg metformin hydrochloride twice daily. JENTADUETO should be given twice daily with meals. Dose escalation should be gradual to reduce the gastrointestinal (GI) side effects associated with metformin use. For available dosage forms and strengths see.
- Recommended starting dose:
- In patients currently not treated with metformin, initiate treatment with 2.5 mg linagliptin/500 mg metformin hydrochloride twice daily
- In patients already treated with metformin, start with 2.5 mg linagliptin and the current dose of metformin taken at each of the two daily meals (e.g., a patient on metformin 1000 mg twice daily would be started on 2.5 mg linagliptin/1000 mg metformin hydrochloride twice daily with meals).
- Patients already treated with linagliptin and metformin individual components may be switched to JENTADUETO containing the same doses of each component.
- No studies have been performed specifically examining the safety and efficacy of JENTADUETO in patients previously treated with other oral antihyperglycemic agents and switched to JENTADUETO. Any change in therapy of type 2 diabetes mellitus should be undertaken with care and appropriate monitoring as changes in glycemic control can occur.
- Concomitant Use with an Insulin Secretagogue (e.g., Sulfonylurea) or with Insulin:
- When JENTADUETO is used in combination with an insulin secretagogue (e.g., sulfonylurea) or with insulin, a lower dose of the insulin secretagogue or insulin may be required to reduce the risk of hypoglycemia.
- JENTADUETO has not been studied in patients with a history of pancreatitis. It is unknown whether patients with a history of pancreatitis are at an increased risk for the development of pancreatitis while using JENTADUETO.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Linagliptin and Metformin hydrochloride in adult patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Linagliptin and Metformin hydrochloride in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Linagliptin and Metformin hydrochloride FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Linagliptin and Metformin hydrochloride in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Linagliptin and Metformin hydrochloride in pediatric patients.
Contraindications
JENTADUETO is contraindicated in patients with:
- Renal impairment (e.g., serum creatinine ≥1.5 mg/dL for men, ≥1.4 mg/dL for women, or abnormal creatinine clearance), which may also result from conditions such as cardiovascular collapse (shock), acute myocardial infarction, and septicemia.
- Acute or chronic metabolic acidosis, including diabetic ketoacidosis. Diabetic ketoacidosis should be treated with insulin.
- A history of hypersensitivity reaction to linagliptin, such as anaphylaxis, angioedema, exfoliative skin conditions, urticaria, or bronchial hyperreactivity.
- Hypersensitivity to metformin
Warnings
|
WARNING: RISK OF LACTIC ACIDOSIS
See full prescribing information for complete Boxed Warning.
Risk of lactic acidosis:
|
5.1 Lactic Acidosis
Metformin
Lactic acidosis is a serious, metabolic complication that can occur due to metformin accumulation during treatment with JENTADUETO and is fatal in approximately 50% of cases. Lactic acidosis may also occur in association with a number of pathophysiologic conditions, including diabetes mellitus, and whenever there is significant tissue hypoperfusion and hypoxemia. Lactic acidosis is characterized by elevated blood lactate levels (>5 mmol/L), decreased blood pH, electrolyte disturbances with an increased anion gap, and an increased lactate/pyruvate ratio. When metformin is implicated as the cause of lactic acidosis, metformin plasma levels of >5 µg/mL are generally found.
The reported incidence of lactic acidosis in patients receiving metformin is approximately 0.03 cases/1000 patient-years, (with approximately 0.015 fatal cases/1000 patient-years). In more than 20,000 patient-years exposure to metformin in clinical trials, there were no reports of lactic acidosis. Reported cases have occurred primarily in diabetic patients with significant renal impairment, including both intrinsic renal disease and renal hypoperfusion, often in the setting of multiple concomitant medical/surgical problems and multiple concomitant medications. Patients with congestive heart failure requiring pharmacologic management, particularly when accompanied by hypoperfusion and hypoxemia due to unstable or acute failure, are at increased risk of lactic acidosis. The risk of lactic acidosis increases with the degree of renal impairment and the patient’s age. The risk of lactic acidosis may, therefore, be significantly decreased by regular monitoring of renal function in patients taking metformin. In particular, treatment of the elderly should be accompanied by careful monitoring of renal function. Metformin treatment should not be initiated in any patient unless measurement of creatinine clearance demonstrates that renal function is not reduced. In addition, metformin should be promptly withheld in the presence of any condition associated with hypoxemia, dehydration, or sepsis. Because impaired hepatic function may significantly limit the ability to clear lactate, metformin should be avoided in patients with clinical or laboratory evidence of hepatic impairment. Patients should be cautioned against excessive alcohol intake when taking metformin, since alcohol potentiates the effects of metformin on lactate metabolism. In addition, metformin should be temporarily discontinued prior to any intravascular radiocontrast study and for any surgical procedure necessitating restricted intake of food or fluids. Use of topiramate, a carbonic anhydrase inhibitor, in epilepsy and migraine prophylaxis may cause dose-dependent metabolic acidosis and may exacerbate the risk of metformin-induced lactic acidosis [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
The onset of lactic acidosis is often subtle, and accompanied by nonspecific symptoms such as malaise, myalgias, respiratory distress, increasing somnolence, and nonspecific abdominal distress. More severe acidosis may be associated with signs such as hypothermia, hypotension, and resistant bradyarrhythmias. Patients should be educated to recognize and promptly report these symptoms. If present, JENTADUETO should be discontinued until lactic acidosis is ruled out. Gastrointestinal symptoms, which are commonly reported during initiation of metformin therapy are less frequently observed in subjects on a chronic, stable, dose of metformin. Gastrointestinal symptoms in subjects on chronic, stable, dose of metformin could be caused by lactic acidosis or other serious disease.
To rule out lactic acidosis, serum electrolytes, ketones, blood glucose, blood pH, lactate levels, and blood metformin levels may be useful. Levels of fasting venous plasma lactate above the upper limit of normal but less than 5 mmol/L in patients taking metformin do not necessarily indicate impending lactic acidosis and may be due to other mechanisms, such as poorly-controlled diabetes or obesity, vigorous physical activity, or technical problems in sample handling.
Lactic acidosis should be suspected in any diabetic patient with metabolic acidosis lacking evidence of ketoacidosis (ketonuria and ketonemia). Lactic acidosis is a medical emergency that must be treated in a hospital setting. In a patient with lactic acidosis who is taking metformin, the drug should be discontinued immediately and supportive measures promptly instituted. Metformin is dialyzable (clearance of up to 170 mL/min under good hemodynamic conditions) and prompt hemodialysis is recommended to remove the accumulated metformin and correct the metabolic acidosis. Such management often results in prompt reversal of symptoms and recovery [see Boxed Warning].
5.2 Pancreatitis
There have been postmarketing reports of acute pancreatitis, including fatal pancreatitis, in patients taking linagliptin. Take careful notice of potential signs and symptoms of pancreatitis. If pancreatitis is suspected, promptly discontinue JENTADUETO and initiate appropriate management. It is unknown whether patients with a history of pancreatitis are at increased risk for the development of pancreatitis while using JENTADUETO.
5.3 Monitoring of Renal Function
Although linagliptin undergoes minimal renal excretion, metformin is known to be substantially excreted by the kidney. The risk of metformin accumulation and lactic acidosis increases with the degree of renal impairment. Therefore, JENTADUETO is contraindicated in patients with renal impairment.
Before initiation of therapy with JENTADUETO and at least annually thereafter, renal function should be assessed and verified to be normal. In patients in whom development of renal impairment is anticipated (e.g., elderly), renal function should be assessed more frequently and JENTADUETO discontinued if evidence of renal impairment is present.
Linagliptin may be continued as a single entity tablet at the same total daily dose of 5 mg if JENTADUETO is discontinued due to evidence of renal impairment. No dose adjustment of linagliptin is recommended in patients with renal impairment.
Use of concomitant medications that may affect renal function or metformin disposition:
Concomitant medication(s) that may affect renal function or result in significant hemodynamic change or interfere with the disposition of metformin should be used with caution [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
Radiological studies and surgical procedures:
Radiologic studies involving the use of intravascular iodinated contrast materials (e.g., intravenous urogram, intravenous cholangiography, angiography, and computed tomography) can lead to acute alteration of renal function and have been associated with lactic acidosis in patients receiving metformin. Therefore, in patients in whom any such study is planned, JENTADUETO should be temporarily discontinued at the time of or prior to the procedure, and withheld for 48 hours subsequent to the procedure and reinstituted only after renal function has been confirmed to be normal.
JENTADUETO should be temporarily discontinued for any surgical procedure (except minor procedures not associated with restricted intake of food and fluids) and should not be restarted until the patient’s oral intake has resumed and renal function has been evaluated as normal.
5.4 Impaired Hepatic Function
Because impaired hepatic function has been associated with some cases of lactic acidosis with metformin therapy, JENTADUETO should generally be avoided in patients with clinical or laboratory evidence of hepatic disease [see Warnings and Precautions (5.1)].
5.5 Use with Medications Known to Cause Hypoglycemia
Linagliptin
Insulin secretagogues and insulin are known to cause hypoglycemia. The use of linagliptin in combination with an insulin secretagogue (e.g., sulfonylurea) was associated with a higher rate of hypoglycemia compared with placebo in a clinical trial [see Adverse Reactions (6.1)]. The use of linagliptin in combination with insulin in subjects with severe renal impairment was associated with a higher rate of hypoglycemia [see Adverse Reactions (6.1)]. Therefore, a lower dose of the insulin secretagogue or insulin may be required to reduce the risk of hypoglycemia when used in combination with JENTADUETO [see Dosage and Administration (2.2)].
Metformin
Hypoglycemia does not occur in patients receiving metformin alone under usual circumstances of use, but could occur when caloric intake is deficient, when strenuous exercise is not compensated by caloric supplementation, or during concomitant use with other glucose-lowering agents (such as SUs and insulin) or ethanol. Elderly, debilitated, or malnourished patients, and those with adrenal or pituitary insufficiency or alcohol intoxication are particularly susceptible to hypoglycemic effects. Hypoglycemia may be difficult to recognize in the elderly, and in people who are taking β-adrenergic blocking drugs.
5.6 Hypersensitivity Reactions
There have been postmarketing reports of serious hypersensitivity reactions in patients treated with linagliptin (one of the components of JENTADUETO). These reactions include anaphylaxis, angioedema, and exfoliative skin conditions. Onset of these reactions occurred within the first 3 months after initiation of treatment with linagliptin, with some reports occurring after the first dose. If a serious hypersensitivity reaction is suspected, discontinue JENTADUETO, assess for other potential causes for the event, and institute alternative treatment for diabetes.
Angioedema has also been reported with other dipeptidyl peptidase-4 (DPP-4) inhibitors. Use caution in a patient with a history of angioedema to another DPP-4 inhibitor because it is unknown whether such patients will be predisposed to angioedema with JENTADUETO.
5.7 Vitamin B12 Levels
In controlled, 29-week clinical trials of metformin, a decrease to subnormal levels of previously normal serum vitamin B12 levels, without clinical manifestations, was observed in approximately 7% of metformin-treated patients. Such decrease, possibly due to interference with B12 absorption from the B12-intrinsic factor complex, is, however, very rarely associated with anemia or neurologic manifestations due to the short duration (<1 year) of the clinical trials. This risk may be more relevant to patients receiving long-term treatment with metformin, and adverse hematologic and neurologic reactions have been reported postmarketing. The decrease in vitamin B12 levels appears to be rapidly reversible with discontinuation of metformin or vitamin B12 supplementation. Measurement of hematologic parameters on an annual basis is advised in patients on JENTADUETO and any apparent abnormalities should be appropriately investigated and managed. Certain individuals (those with inadequate vitamin B12 or calcium intake or absorption) appear to be predisposed to developing subnormal vitamin B12 levels. In these patients, routine serum vitamin B12 measurement at 2- to 3-year intervals may be useful.
5.8 Alcohol Intake
Alcohol is known to potentiate the effect of metformin on lactate metabolism. Patients, therefore, should be warned against excessive alcohol intake while receiving JENTADUETO [see Warnings and Precautions (5.1)].
5.9 Hypoxic States
Cardiovascular collapse (shock) from whatever cause (e.g., acute congestive heart failure, acute myocardial infarction, and other conditions characterized by hypoxemia) have been associated with lactic acidosis and may also cause prerenal azotemia. When such events occur in patients on JENTADUETO therapy, the drug should be promptly discontinued [see Warnings and Precautions (5.1)].
5.10 Macrovascular Outcomes
There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with linagliptin or metformin or any other antidiabetic drug.
Adverse Reactions
Clinical Trials Experience
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Linagliptin/Metformin
The safety of concomitantly administered linagliptin (daily dose 5 mg) and metformin (mean daily dose of approximately 1800 mg) has been evaluated in 2816 patients with type 2 diabetes mellitus treated for ≥12 weeks in clinical trials.
Three placebo-controlled studies with linagliptin + metformin were conducted: 2 studies were 24 weeks in duration, 1 study was 12 weeks in duration. In the 3 placebo-controlled clinical studies, adverse events which occurred in ≥5% of patients receiving linagliptin + metformin (n=875) and were more common than in patients given placebo + metformin (n=539) included nasopharyngitis (5.7% vs 4.3%).
In a 24-week factorial design study, adverse events reported in ≥5% of patients receiving linagliptin + metformin and were more common than in patients given placebo are shown in Table 1.
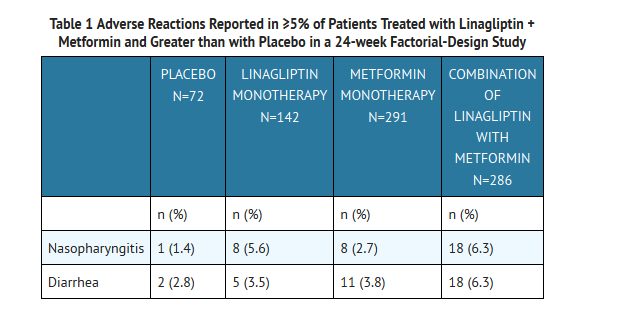
Other adverse reactions reported in clinical studies with treatment of linagliptin + metformin were hypersensitivity (e.g., urticaria, angioedema, or bronchial hyperreactivity), cough, decreased appetite, nausea, vomiting, pruritus, and pancreatitis.
Linagliptin
Adverse reactions reported in ≥2% of patients treated with linagliptin 5 mg and more commonly than in patients treated with placebo included: nasopharyngitis (7.0% vs 6.1%), diarrhea (3.3% vs 3.0%), and cough (2.1% vs 1.4%).
Rates for other adverse reactions for linagliptin 5 mg vs placebo when linagliptin was used in combination with specific anti-diabetic agents were: urinary tract infection (3.1% vs 0%) and hypertriglyceridemia (2.4% vs 0%) when linagliptin was used as add-on to sulfonylurea; hyperlipidemia (2.7% vs 0.8%) and weight increased (2.3% vs 0.8%) when linagliptin was used as add-on to pioglitazone; and constipation (2.1% vs 1%) when linagliptin was used as add-on to basal insulin therapy.
Other adverse reactions reported in clinical studies with treatment of linagliptin monotherapy were hypersensitivity (e.g., urticaria, angioedema, localized skin exfoliation, or bronchial hyperreactivity) and myalgia. In the clinical trial program, pancreatitis was reported in 15.2 cases per 10,000 patient year exposure while being treated with linagliptin compared with 3.7 cases per 10,000 patient year exposure while being treated with comparator (placebo and active comparator, sulfonylurea). Three additional cases of pancreatitis were reported following the last administered dose of linagliptin.
Metformin
The most common adverse reactions due to initiation of metformin are diarrhea, nausea/vomiting, flatulence, asthenia, indigestion, abdominal discomfort, and headache.
Long-term treatment with metformin has been associated with a decrease in vitamin B12 absorption which may very rarely result in clinically significant vitamin B12 deficiency (e.g., megaloblastic anemia) [see Warnings and Precautions (5.5)].
Hypoglycemia Linagliptin/Metformin
In a 24-week factorial design study, hypoglycemia was reported in 4 (1.4%) of 286 subjects treated with linagliptin + metformin, 6 (2.1%) of 291 subjects treated with metformin, and 1 (1.4%) of 72 subjects treated with placebo. When linagliptin was administered in combination with metformin and a sulfonylurea, 181 (22.9%) of 792 patients reported hypoglycemia compared with 39 (14.8%) of 263 patients administered placebo in combination with metformin and sulfonylurea. Adverse reactions of hypoglycemia were based on all reports of hypoglycemia. A concurrent glucose measurement was not required or was normal in some patients. Therefore, it is not possible to conclusively determine that all these reports reflect true hypoglycemia.
Linagliptin
In the study of patients receiving linagliptin as add-on therapy to a stable dose of insulin for up to 52 weeks (n=1261), no significant difference in the incidence of investigator reported hypoglycemia, defined as all symptomatic or asymptomatic episodes with a self-measured blood glucose ≤70 mg/dL, was noted between the linagliptin- (31.4%) and placebo- (32.9%) treated groups.
Use in Renal Impairment
Linagliptin was compared to placebo as add-on to pre-existing antidiabetic therapy over 52 weeks in 133 patients with severe renal impairment (estimated GFR <30 mL/min). For the initial 12 weeks of the study, background antidiabetic therapy was kept stable and included insulin, sulfonylurea, glinides, and pioglitazone. For the remainder of the trial, dose adjustments in antidiabetic background therapy were allowed.
In general, the incidence of adverse events including severe hypoglycemia was similar to those reported in other linagliptin trials. The observed incidence of hypoglycemia was higher (linagliptin, 63% compared to placebo, 49%) due to an increase in asymptomatic hypoglycemic events especially during the first 12 weeks when background glycemic therapies were kept stable. Ten linagliptin-treated patients (15%) and 11 placebo-treated patients (17%) reported at least one episode of confirmed symptomatic hypoglycemia (accompanying finger stick glucose ≤54 mg/dL). During the same time period, severe hypoglycemic events, defined as an event requiring the assistance of another person to actively administer carbohydrate, glucagon or other resuscitative actions, were reported in 3 (4.4%) linagliptin-treated patients and 3 (4.6%) placebo-treated patients. Events that were considered life-threatening or required hospitalization were reported in 2 (2.9%) patients on linagliptin and 1 (1.5%) patient on placebo.
Renal function as measured by mean eGFR and creatinine clearance did not change over 52 weeks’ treatment compared to placebo.
Laboratory Tests
Changes in laboratory findings were similar in patients treated with linagliptin + metformin compared to patients treated with placebo + metformin. Changes in laboratory values that occurred more frequently in the linagliptin + metformin group and ≥1% more than in the placebo group were not detected.
No clinically meaningful changes in vital signs were observed in patients treated with linagliptin.
Postmarketing Experience
- The following adverse reactions have been identified during postapproval use of linagliptin. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Acute pancreatitis, including fatal pancreatitis
- Hypersensitivity reactions including anaphylaxis, angioedema, and exfoliative skin conditions
- Rash
Drug Interactions
7.1 Drug Interactions with Metformin
Cationic Drugs
Cationic drugs (e.g., amiloride, digoxin, morphine, procainamide, quinidine, quinine, ranitidine, triamterene, trimethoprim, or vancomycin) that are eliminated by renal tubular secretion theoretically have the potential for interaction with metformin by competing for common renal tubular transport systems. Although such interactions remain theoretical (except for cimetidine), careful patient monitoring and dose adjustment of JENTADUETO and/or the interfering drug is recommended in patients who are taking cationic medications that are excreted via the proximal renal tubular secretory system [see Warnings and Precautions (5.3) and Clinical Pharmacology (12.3)].
Carbonic Anhydrase Inhibitors
Topiramate or other carbonic anhydrase inhibitors (e.g., zonisamide, acetazolamide or dichlorphenamide) frequently decrease serum bicarbonate and induce non-anion gap, hyperchloremic metabolic acidosis. Concomitant use of these drugs may induce metabolic acidosis. Use these drugs with caution in patients treated with JENTADUETO, as the risk of lactic acidosis may increase [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].
7.2 Drug Interactions with Linagliptin
Inducers of P-glycoprotein and CYP3A4 Enzymes
Rifampin decreased linagliptin exposure, suggesting that the efficacy of linagliptin may be reduced when administered in combination with a strong P-gp inducer or CYP 3A4 inducer. As JENTADUETO is a fixed-dose combination of linagliptin and metformin, use of alternative treatments (not containing linagliptin) is strongly recommended when concomitant treatment with a strong P-gp or CYP 3A4 inducer is necessary [see Clinical Pharmacology (12.3)].
7.3 Drugs Affecting Glycemic Control
Certain drugs tend to produce hyperglycemia and may lead to loss of glycemic control. These drugs include the thiazides and other diuretics, corticosteroids, phenothiazines, thyroid products, estrogens, oral contraceptives, phenytoin, nicotinic acid, sympathomimetics, calcium channel blocking drugs, and isoniazid. When such drugs are administered to a patient receiving JENTADUETO, the patient should be closely observed to maintain adequate glycemic control [see Clinical Pharmacology (12.3)]. When such drugs are withdrawn from a patient receiving JENTADUETO, the patient should be observed closely for hypoglycemia.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): B Pregnancy Category B
JENTADUETO
There are no adequate and well controlled studies in pregnant women with JENTADUETO or its individual components, and some clinical data is available for metformin which indicate that the risk for major malformations was not increased when metformin is taken during the first trimester in pregnancy. In addition, metformin was not associated with increased perinatal complications. Nevertheless, because these clinical data cannot rule out the possibility of harm, JENTADUETO should be used during pregnancy only if clearly needed.
JENTADUETO was not teratogenic when administered to Wistar Han rats during the period of organogenesis at doses similar to clinical exposure. At higher maternally toxic doses (9 and 23 times the clinical dose based on exposure), the metformin component of the combination was associated with an increased incidence of fetal rib and scapula malformations.
Linagliptin
Linagliptin was not teratogenic when administered to pregnant Wistar Han rats and Himalayan rabbits during the period of organogenesis at doses up to 240 mg/kg and 150 mg/kg, respectively. These doses represent approximately 943 times the clinical dose in rats and 1943 times the clinical dose in rabbits, based on exposure. No functional, behavioral, or reproductive toxicity was observed in offspring of female Wistar Han rats when administered linagliptin from gestation day 6 to lactation day 21 at a dose 49 times the maximum recommended human dose, based on exposure.
Linagliptin crosses the placenta into the fetus following oral dosing in pregnant rats and rabbits.
Metformin Hydrochloride
Metformin has been studied for embryo-fetal effects in 2 rat strains and in rabbits. Metformin was not teratogenic in Sprague Dawley rats up to 600 mg/kg or in Wistar Han rats up to 200 mg/kg (2-3 times the clinical dose based on body surface area or exposure, respectively). At higher maternally toxic doses (9 and 23 times the clinical dose based on exposure), an increased incidence of rib and scapula skeletal malformations was observed in the Wistar Han strain. Metformin was not teratogenic in rabbits at doses up to 140 mg/kg (similar to clinical dose based on body surface area).
Metformin administered to female Sprague Dawley rats from gestation day 6 to lactation day 21 up to 600 mg/kg/day (2 times the maximum clinical dose based on body surface area) had no effect on prenatal or postnatal development of offspring.
Metformin crosses the placenta into the fetus in rats and humans.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Linagliptin and Metformin hydrochloride in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Linagliptin and Metformin hydrochloride during labor and delivery.
Nursing Mothers
No studies in lactating animals have been conducted with the combined components of JENTADUETO. In studies performed with the individual components, both linagliptin and metformin were secreted in the milk of lactating rats. It is not known whether linagliptin is excreted in human milk. Metformin is excreted in human milk in low concentrations. Because the potential for hypoglycemia in nursing infants may exist, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness of JENTADUETO in pediatric patients under 18 years of age have not been established.
Geriatic Use
Linagliptin is minimally excreted by the kidney; however, metformin is substantially excreted by the kidney. Considering that aging can be associated with reduced renal function, JENTADUETO should be used with caution as age increases [see Warnings and Precautions (5.1, 5.3) and Clinical Pharmacology (12.3)].
Linagliptin
There were 4040 type 2 diabetes patients treated with linagliptin 5 mg from 15 clinical trials of linagliptin; 1085 (27%) patients were 65 years and over, while 131 (3%) were 75 years and over. Of these patients, 2566 were enrolled in 12 double-blind placebo-controlled studies; 591 (23%) were 65 years and over, while 82 (3%) were 75 years and over. No overall differences in safety or effectiveness were observed between patients 65 years and over and younger patients. Therefore, no dose adjustment is recommended in the elderly population. While clinical studies of linagliptin have not identified differences in response between the elderly and younger patients, greater sensitivity of some older individuals cannot be ruled out.
Metformin
Controlled clinical studies of metformin did not include sufficient numbers of elderly patients to determine whether they respond differently from younger patients, although other reported clinical experience has not identified differences in responses between the elderly and young patients. The initial and maintenance dosing of metformin should be conservative in patients with advanced age, due to the potential for decreased renal function in this population. Any dose adjustment should be based on a careful assessment of renal function [see Contraindications (4), Warnings and Precautions (5.3), and Clinical Pharmacology (12.3)].
Gender
There is no FDA guidance on the use of Linagliptin and Metformin hydrochloride with respect to specific gender populations.
Race
There is no FDA guidance on the use of Linagliptin and Metformin hydrochloride with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Linagliptin and Metformin hydrochloride in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Linagliptin and Metformin hydrochloride in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Linagliptin and Metformin hydrochloride in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Linagliptin and Metformin hydrochloride in patients who are immunocompromised.
Administration and Monitoring
Administration
Recommended Dosing
- The dosage of JENTADUETO should be individualized on the basis of both effectiveness and tolerability, while not exceeding the maximum recommended dose of 2.5 mg linagliptin/1000 mg metformin hydrochloride twice daily. JENTADUETO should be given twice daily with meals. Dose escalation should be gradual to reduce the gastrointestinal (GI) side effects associated with metformin use. For available dosage forms and strengths see.
- Recommended starting dose:
- In patients currently not treated with metformin, initiate treatment with 2.5 mg linagliptin/500 mg metformin hydrochloride twice daily
- In patients already treated with metformin, start with 2.5 mg linagliptin and the current dose of metformin taken at each of the two daily meals (e.g., a patient on metformin 1000 mg twice daily would be started on 2.5 mg linagliptin/1000 mg metformin hydrochloride twice daily with meals).
- Patients already treated with linagliptin and metformin individual components may be switched to JENTADUETO containing the same doses of each component.
- No studies have been performed specifically examining the safety and efficacy of JENTADUETO in patients previously treated with other oral antihyperglycemic agents and switched to JENTADUETO. Any change in therapy of type 2 diabetes mellitus should be undertaken with care and appropriate monitoring as changes in glycemic control can occur.
Concomitant Use with an Insulin Secretagogue (e.g., Sulfonylurea) or with Insulin
- When JENTADUETO is used in combination with an insulin secretagogue (e.g., sulfonylurea) or with insulin, a lower dose of the insulin secretagogue or insulin may be required to reduce the risk of hypoglycemia.
Dosage forms and strengths
- JENTADUETO is a combination of linagliptin and metformin hydrochloride. JENTADUETO tablets are available in the following dosage forms and strengths:
- 2.5 mg linagliptin/500 mg metformin hydrochloride tablets are light yellow, oval, biconvex tablets debossed with “D2/500” on one side and the Boehringer Ingelheim logo on the other side
- 2.5 mg linagliptin/850 mg metformin hydrochloride tablets are light orange, oval, biconvex tablets debossed with “D2/850” on one side and the Boehringer Ingelheim logo on the other side
- 2.5 mg linagliptin/1000 mg metformin hydrochloride tablets are light pink, oval, biconvex tablets debossed with “D2/1000” on one side and the Boehringer Ingelheim logo on the other side
Monitoring
There is limited information regarding Linagliptin and Metformin hydrochloride Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Linagliptin and Metformin hydrochloride and IV administrations.
Overdosage
- In the event of an overdose with JENTADUETO, contact the Poison Control Center. Employ the usual supportive measures (e.g., remove unabsorbed material from the gastrointestinal tract, employ clinical monitoring, and institute supportive treatment) as dictated by the patient’s clinical status. Removal of linagliptin by hemodialysis or peritoneal dialysis is unlikely. However, metformin is dialyzable with a clearance of up to 170 mL/min under good hemodynamic conditions. Therefore, hemodialysis may be useful partly for removal of accumulated metformin from patients in whom JENTADUETO overdosage is suspected.
Linagliptin
During controlled clinical trials in healthy subjects, with single doses of up to 600 mg of linagliptin (equivalent to 120 times the recommended daily dose), there were no dose-related clinical adverse drug reactions. There is no experience with doses above 600 mg in humans.
Metformin
Overdose of metformin has occurred, including ingestion of amounts greater than 50 grams. Hypoglycemia was reported in approximately 10% of cases, but no causal association with metformin has been established. Lactic acidosis has been reported in approximately 32% of metformin overdose cases [see Boxed Warning and Warnings and Precautions (5.1)].
Pharmacology
There is limited information regarding Linagliptin and Metformin hydrochloride Pharmacology in the drug label.
Mechanism of Action
JENTADUETO
JENTADUETO combines 2 antihyperglycemic agents with complementary mechanisms of action to improve glycemic control in patients with type 2 diabetes mellitus: linagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor, and metformin, a member of the biguanide class.
Linagliptin
Linagliptin is an inhibitor of DPP-4, an enzyme that degrades the incretin hormones glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). Thus, linagliptin increases the concentrations of active incretin hormones, stimulating the release of insulin in a glucose-dependent manner and decreasing the levels of glucagon in the circulation. Both incretin hormones are involved in the physiological regulation of glucose homeostasis. Incretin hormones are secreted at a low basal level throughout the day and levels rise immediately after meal intake. GLP-1 and GIP increase insulin biosynthesis and secretion from pancreatic beta cells in the presence of normal and elevated blood glucose levels. Furthermore, GLP-1 also reduces glucagon secretion from pancreatic alpha cells, resulting in a reduction in hepatic glucose output.
Metformin
Metformin is an antihyperglycemic agent which improves glucose tolerance in patients with type 2 diabetes mellitus, lowering both basal and postprandial plasma glucose. Its pharmacologic mechanisms of action are different from other classes of oral antihyperglycemic agents. Metformin decreases hepatic glucose production, decreases intestinal absorption of glucose, and improves insulin sensitivity by increasing peripheral glucose uptake and utilization. Unlike SUs, metformin does not produce hypoglycemia in either patients with type 2 diabetes mellitus or normal subjects (except in special circumstances) [see Warnings and Precautions (5.10)] and does not cause hyperinsulinemia. With metformin therapy, insulin secretion remains unchanged while fasting insulin levels and day-long plasma insulin response may actually decrease.
Structure
JENTADUETO tablets contain 2 oral antihyperglycemic drugs used in the management of type 2 diabetes mellitus: linagliptin and metformin hydrochloride.
Linagliptin
Linagliptin is an orally-active inhibitor of the dipeptidyl peptidase-4 (DPP-4) enzyme.
Linagliptin is described chemically as 1H-Purine-2,6-dione, 8-[(3R)-3-amino-1-piperidinyl]-7-(2-butyn-1-yl)-3,7-dihydro-3-methyl-1-[(4-methyl-2-quinazolinyl)methyl]-
The empirical formula is C25H28N8O2 and the molecular weight is 472.54 g/mol. The structural formula is: Linagliptin structure
Linagliptin is a white to yellowish, not or only slightly hygroscopic solid substance. It is very slightly soluble in water (0.9 mg/mL). Linagliptin is soluble in methanol (ca. 60 mg/mL), sparingly soluble in ethanol (ca. 10 mg/mL), very slightly soluble in isopropanol (<1 mg/mL), and very slightly soluble in acetone (ca. 1 mg/mL).
Metformin Hydrochloride
Metformin hydrochloride (N,N-dimethylimidodicarbonimidic diamide hydrochloride) is not chemically or pharmacologically related to any other classes of oral antihyperglycemic agents. Metformin hydrochloride is a white to off-white crystalline compound with a molecular formula of C4H11N5•HCl and a molecular weight of 165.63. Metformin hydrochloride is freely soluble in water and is practically insoluble in acetone, ether, and chloroform. The pKa of metformin is 12.4. The pH of a 1% aqueous solution of metformin hydrochloride is 6.68. The structural formula is:
Metformin structure
JENTADUETO
JENTADUETO is available for oral administration as tablets containing 2.5 mg linagliptin and 500 mg metformin hydrochloride (JENTADUETO 2.5 mg/500 mg), 850 mg metformin hydrochloride (JENTADUETO 2.5 mg/850 mg) or 1000 mg metformin hydrochloride (JENTADUETO 2.5 mg/1000 mg). Each film-coated tablet of JENTADUETO contains the following inactive ingredients: arginine, corn starch, copovidone, colloidal silicon dioxide, magnesium stearate, titanium dioxide, propylene glycol, hypromellose, talc, yellow ferric oxide (2.5 mg/500 mg; 2.5 mg/850 mg) and/or red ferric oxide (2.5 mg/850 mg; 2.5 mg/1000 mg).
Pharmacodynamics
Linagliptin
Linagliptin binds to DPP-4 in a reversible manner and increases the concentrations of incretin hormones. Linagliptin glucose-dependently increases insulin secretion and lowers glucagon secretion, thus resulting in a better regulation of the glucose homeostasis. Linagliptin binds selectively to DPP-4 and selectively inhibits DPP-4, but not DPP-8 or DPP-9 activity in vitro at concentrations approximating therapeutic exposures.
Cardiac Electrophysiology
In a randomized, placebo-controlled, active-comparator, 4-way crossover study, 36 healthy subjects were administered a single oral dose of linagliptin 5 mg, linagliptin 100 mg (20 times the recommended dose), moxifloxacin, and placebo. No increase in QTc was observed with either the recommended dose of 5 mg or the 100-mg dose. At the 100-mg dose, peak linagliptin plasma concentrations were approximately 38-fold higher than the peak concentrations following a 5-mg dose.
Pharmacokinetics
JENTADUETO
The results of a bioequivalence study in healthy subjects demonstrated that JENTADUETO (linagliptin/metformin hydrochloride) 2.5 mg/500 mg, 2.5 mg/850 mg, and 2.5 mg/1000 mg combination tablets are bioequivalent to coadministration of corresponding doses of linagliptin and metformin as individual tablets. Administration of linagliptin 2.5 mg/metformin hydrochloride 1000 mg fixed-dose combination with food resulted in no change in overall exposure of linagliptin. There was no change in metformin AUC; however, mean peak serum concentration of metformin was decreased by 18% when administered with food. A delayed time-to-peak serum concentrations by 2 hours was observed for metformin under fed conditions. These changes are not likely to be clinically significant.
Absorption
Linagliptin
The absolute bioavailability of linagliptin is approximately 30%. Following oral administration, plasma concentrations of linagliptin decline in at least a biphasic manner with a long terminal half-life (>100 hours), related to the saturable binding of linagliptin to DPP-4. However, the prolonged elimination does not contribute to the accumulation of the drug. The effective half-life for accumulation of linagliptin, as determined from oral administration of multiple doses of linagliptin 5 mg, is approximately 12 hours. After once-daily dosing, steady state plasma concentrations of linagliptin 5 mg are reached by the third dose, and Cmax and AUC increased by a factor of 1.3 at steady-state compared with the first dose. Plasma AUC of linagliptin increased in a less than dose-proportional manner in the dose range of 1 to 10 mg. The pharmacokinetics of linagliptin is similar in healthy subjects and in patients with type 2 diabetes.
Metformin
The absolute bioavailability of a metformin hydrochloride 500-mg tablet given under fasting conditions is approximately 50% to 60%. Studies using single oral doses of metformin tablets 500 mg to 1500 mg, and 850 mg to 2550 mg, indicate that there is a lack of dose proportionality with increasing doses, which is due to decreased absorption rather than an alteration in elimination.
Distribution
Linagliptin
The mean apparent volume of distribution at steady state following a single intravenous dose of linagliptin 5 mg to healthy subjects is approximately 1110 L, indicating that linagliptin extensively distributes to the tissues. Plasma protein binding of linagliptin is concentration-dependent decreasing from about 99% at 1 nmol/L to 75% to 89% at ≥30 nmol/L, reflecting saturation of binding to DPP-4 with increasing concentration of linagliptin. At high concentrations, where DPP-4 is fully saturated, 70% to 80% of linagliptin remains bound to plasma proteins and 20% to 30% is unbound in plasma. Plasma binding is not altered in patients with renal or hepatic impairment.
Metformin
The apparent volume of distribution (V/F) of metformin following single oral doses of immediate-release metformin hydrochloride tablets 850 mg averaged 654±358 L. Metformin is negligibly bound to plasma proteins, in contrast to SUs, which are more than 90% protein bound. Metformin partitions into erythrocytes, most likely as a function of time. At usual clinical doses and dosing schedules of metformin tablets, steady-state plasma concentrations of metformin are reached within 24 to 48 hours and are generally <1 mcg/mL. During controlled clinical trials of metformin, maximum metformin plasma levels did not exceed 5 mcg/mL, even at maximum doses.
Metabolism
Linagliptin
Following oral administration, the majority (about 90%) of linagliptin is excreted unchanged, indicating that metabolism represents a minor elimination pathway. A small fraction of absorbed linagliptin is metabolized to a pharmacologically inactive metabolite, which shows a steady-state exposure of 13.3% relative to linagliptin.
Metformin
Intravenous single-dose studies in normal subjects demonstrate that metformin is excreted unchanged in the urine and does not undergo hepatic metabolism (no metabolites have been identified in humans) nor biliary excretion.
Excretion
Linagliptin
Following administration of an oral [14C]linagliptin dose to healthy subjects, approximately 85% of the administered radioactivity was eliminated via the enterohepatic system (80%) or urine (5%) within 4 days of dosing. Renal clearance at steady state was approximately 70 mL/min.
Metformin
Renal clearance is approximately 3.5 times greater than creatinine clearance, which indicates that tubular secretion is the major route of metformin elimination. Following oral administration, approximately 90% of the absorbed drug is eliminated via the renal route within the first 24 hours, with a plasma elimination half-life of approximately 6.2 hours. In blood, the elimination half-life is approximately 17.6 hours, suggesting that the erythrocyte mass may be a compartment of distribution.
Specific Populations
Renal Impairment
JENTADUETO: Studies characterizing the pharmacokinetics of linagliptin and metformin after administration of JENTADUETO in renally impaired patients have not been performed. Since metformin is contraindicated in patients with renal impairment, use of JENTADUETO is also contraindicated in patients with renal impairment (e.g., serum creatinine ≥1.5 mg/dL [males] or ≥1.4 mg/dL [females], or abnormal creatinine clearance) [see Contraindications (4) and Warnings and Precautions (5.3)].
Linagliptin: Under steady-state conditions, linagliptin exposure in patients with mild renal impairment was comparable to healthy subjects. In patients with moderate renal impairment under steady-state conditions, mean exposure of linagliptin increased (AUCτ,ss by 71% and Cmax by 46%) compared with healthy subjects. This increase was not associated with a prolonged accumulation half-life, terminal half-life, or an increased accumulation factor. Renal excretion of linagliptin was below 5% of the administered dose and was not affected by decreased renal function.
Patients with type 2 diabetes mellitus and severe renal impairment showed steady-state exposure approximately 40% higher than that of patients with type 2 diabetes mellitus and normal renal function (increase in AUC by 42% and Cmax by 35%). For both type 2 diabetes mellitus groups, renal excretion was below 7% of the administered dose.
Metformin: In patients with decreased renal function (based on measured creatinine clearance), the plasma and blood half-life of metformin is prolonged and the renal clearance is decreased in proportion to the decrease in creatinine clearance [see Contraindications (4) and Warnings and Precautions (5.3)].
Hepatic Impairment
JENTADUETO: Studies characterizing the pharmacokinetics of linagliptin and metformin after administration of JENTADUETO in hepatically impaired patients have not been performed. However, use of metformin alone in patients with hepatic impairment has been associated with some cases of lactic acidosis. Therefore, use of JENTADUETO is not recommended in patients with hepatic impairment [see Warnings and Precautions (5.4)].
Linagliptin: In patients with mild hepatic impairment (Child-Pugh class A) steady-state exposure (AUCτ,ss) of linagliptin was approximately 25% lower and Cmax,ss was approximately 36% lower than in healthy subjects. In patients with moderate hepatic impairment (Child-Pugh class B), AUCss of linagliptin was about 14% lower and Cmax,ss was approximately 8% lower than in healthy subjects. Patients with severe hepatic impairment (Child-Pugh class C) had comparable exposure of linagliptin in terms of AUC0-24 and approximately 23% lower Cmax compared with healthy subjects. Reductions in the pharmacokinetic parameters seen in patients with hepatic impairment did not result in reductions in DPP-4 inhibition.
Metformin hydrochloride: No pharmacokinetic studies of metformin have been conducted in patients with hepatic impairment.
Body Mass Index (BMI)/Weight
Linagliptin: BMI/Weight had no clinically meaningful effect on the pharmacokinetics of linagliptin based on a population pharmacokinetic analysis.
Gender
Linagliptin: Gender had no clinically meaningful effect on the pharmacokinetics of linagliptin based on a population pharmacokinetic analysis.
Metformin hydrochloride: Metformin pharmacokinetic parameters did not differ significantly between normal subjects and patients with type 2 diabetes mellitus when analyzed according to gender. Similarly, in controlled clinical studies in patients with type 2 diabetes mellitus, the antihyperglycemic effect of metformin was comparable in males and females.
Geriatric
JENTADUETO: Studies characterizing the pharmacokinetics of linagliptin and metformin after administration of JENTADUETO in geriatric patients have not been performed. Based on the metformin component, JENTADUETO treatment should not be initiated in patients ≥80 years of age unless measurement of creatinine clearance demonstrates that renal function is not reduced [see Warnings and Precautions (5.1, 5.3) and Use in Specific Populations (8.5)].
Linagliptin: Age did not have a clinically meaningful impact on the pharmacokinetics of linagliptin based on a population pharmacokinetic analysis.
Metformin hydrochloride: Limited data from controlled pharmacokinetic studies of metformin in healthy elderly subjects suggest that total plasma clearance of metformin is decreased, the half-life is prolonged, and Cmax is increased, compared with healthy young subjects. From these data, it appears that the change in metformin pharmacokinetics with aging is primarily accounted for by a change in renal function.
Pediatric
Studies characterizing the pharmacokinetics of linagliptin and metformin after administration of JENTADUETO in pediatric patients have not yet been performed.
Race
Linagliptin: Race had no clinically meaningful effect on the pharmacokinetics of linagliptin based on available pharmacokinetic data, including subjects of White, Hispanic, Black, and Asian racial groups.
Metformin hydrochloride: No studies of metformin pharmacokinetic parameters according to race have been performed. In controlled clinical studies of metformin in patients with type 2 diabetes mellitus, the antihyperglycemic effect was comparable in Caucasians (n=249), Blacks (n=51), and Hispanics (n=24).
Drug Interactions
Pharmacokinetic drug interaction studies with JENTADUETO have not been performed; however, such studies have been conducted with the individual components of JENTADUETO (linagliptin and metformin hydrochloride).
Linagliptin
In vitro Assessment of Drug Interactions
Linagliptin is a weak to moderate inhibitor of CYP isozyme CYP3A4, but does not inhibit other CYP isozymes and is not an inducer of CYP isozymes, including CYP1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, and 4A11.
Linagliptin is a P-glycoprotein (P-gp) substrate, and inhibits P-gp mediated transport of digoxin at high concentrations. Based on these results and in vivo drug interaction studies, linagliptin is considered unlikely to cause interactions with other P-gp substrates at therapeutic concentrations.
In vivo Assessment of Drug Interactions
Strong inducers of CYP3A4 or P-gp (e.g., rifampin) decrease exposure to linagliptin to subtherapeutic and likely ineffective concentrations. For patients requiring use of such drugs, an alternative to linagliptin is strongly recommended. In vivo studies indicated evidence of a low propensity for causing drug interactions with substrates of CYP3A4, CYP2C9, CYP2C8, P-gp, and OCT. No dose adjustment of linagliptin is recommended based on results of the described pharmacokinetic studies.
Nonclinical Toxicology
There is limited information regarding Linagliptin and Metformin hydrochloride Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Linagliptin and Metformin hydrochloride Clinical Studies in the drug label.
How Supplied
There is limited information regarding Linagliptin and Metformin hydrochloride How Supplied in the drug label.
Storage
There is limited information regarding Linagliptin and Metformin hydrochloride Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Linagliptin and Metformin hydrochloride |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Linagliptin and Metformin hydrochloride |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Linagliptin and Metformin hydrochloride Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Linagliptin and Metformin hydrochloride interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Linagliptin and Metformin hydrochloride Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Linagliptin and Metformin hydrochloride Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.