Lactam: Difference between revisions
Jump to navigation
Jump to search
m (Robot: Automated text replacement (-{{SIB}} + & -{{EH}} + & -{{EJ}} + & -{{Editor Help}} + & -{{Editor Join}} +)) |
m (Robot: Automated text replacement (-{{WikiDoc Cardiology Network Infobox}} +, -<references /> +{{reflist|2}}, -{{reflist}} +{{reflist|2}})) |
||
| Line 23: | Line 23: | ||
==References== | ==References== | ||
{{reflist|2}} | |||
==External links== | ==External links== | ||
Latest revision as of 18:59, 4 September 2012
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
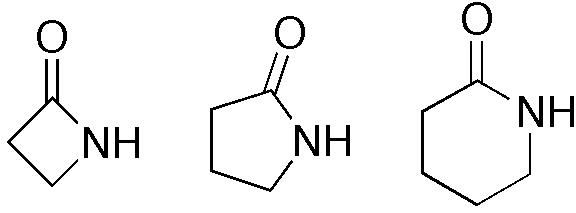
A lactam (the noun is a portmanteau of the words lactone + amide) is a cyclic amide. Prefixes may indicate the ring size: β-lactam (4-membered), γ-lactam (5-membered), δ-lactam (6-membered ring). That order in the nomenclature is because beta β, gamma γ and delta δ are the second, third and fourth letters in the alphabetical order of the Greek alphabet, respectively.
Synthesis
General synthetic methods exist for the organic synthesis of lactams.
- Lactams form by the acid-catalyzed rearrangement of oximes in the Beckmann rearrangement.
- Lactams form from cyclic ketones and ammonia in the Schmidt reaction.
- lactams form from cyclisation of amino acids.
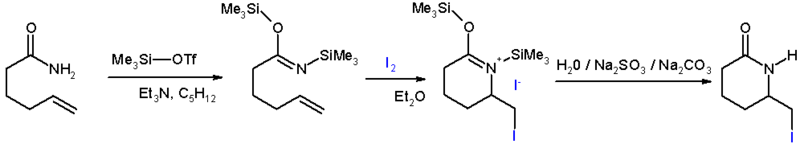
- In iodolactamization [1] an iminium ion reacts with an halonium ion formed in situ by reaction of an alkene with iodine.
- Lactams form by copper catalyzed 1,3-dipolar cycloaddition of alkynes and nitrones in the Kinugasa reaction
Reactions
- Lactams can polymerize to polyamides.
See also
- β-lactam with a four-membered ring found in beta-lactam antibiotics. Penicillin, considered the most famous antibiotic, is a β-lactam antibiotic.
- Lactone, a cyclic ester.
- Caprolactam
References
- ↑ Spencer Knapp, Frank S. Gibson Organic Syntheses, Coll. Vol. 9, p.516 (1998); Vol. 70, p.101 (1992) Online article