Krukenberg tumor: Difference between revisions
No edit summary |
No edit summary |
||
| Line 15: | Line 15: | ||
__NOTOC__ | __NOTOC__ | ||
{{SI}} | {{SI}} | ||
{{CMG}} {{AE}} | {{CMG}} {{AE}} {{STM}} | ||
{{SK}} | {{SK}} carcinoma mucocellulare; Synonym 2; Synonym 3 | ||
==Overview== | ==Overview== | ||
Krukenberg's tumor is a rare metastatic signet ring cell adenocarcinoma of the ovary.<ref name="pmid17076540">{{cite journal| author=Al-Agha OM, Nicastri AD| title=An in-depth look at Krukenberg tumor: an overview. | journal=Arch Pathol Lab Med | year= 2006 | volume= 130 | issue= 11 | pages= 1725-30 | pmid=17076540 | doi=10.1043/1543-2165(2006)130[1725:AILAKT]2.0.CO;2 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17076540 }} </ref> | Krukenberg's tumor is a rare metastatic signet ring cell adenocarcinoma of the ovary.<ref name="pmid17076540">{{cite journal| author=Al-Agha OM, Nicastri AD| title=An in-depth look at Krukenberg tumor: an overview. | journal=Arch Pathol Lab Med | year= 2006 | volume= 130 | issue= 11 | pages= 1725-30 | pmid=17076540 | doi=10.1043/1543-2165(2006)130[1725:AILAKT]2.0.CO;2 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17076540 }} </ref> | ||
==Historical Perspective== | ==Historical Perspective== | ||
*Krukenberg's tumor was first | *Krukenberg's tumor was first described as a new type of primary ovarian malignancy by Friedrich Ernst Krukenberg (1871–1946), a German gynecologist and pathologist, in 1896 which was later confirmed to be of metastatic gastrointestinal tract origin.<ref name="pmid17076540">{{cite journal| author=Al-Agha OM, Nicastri AD| title=An in-depth look at Krukenberg tumor: an overview. | journal=Arch Pathol Lab Med | year= 2006 | volume= 130 | issue= 11 | pages= 1725-30 | pmid=17076540 | doi=10.1043/1543-2165(2006)130[1725:AILAKT]2.0.CO;2 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17076540 }} </ref> | ||
==Classification== | ==Classification== | ||
* | *Krukenberg's tumor may be classified according to [classification method] into [number] subtypes/groups: | ||
:*[group1] | :*[group1] | ||
:*[group2] | :*[group2] | ||
| Line 33: | Line 35: | ||
==Pathophysiology== | ==Pathophysiology== | ||
*The majority of Krukenberg’s tumors are bilateral.<ref name="pmid17076540">{{cite journal| author=Al-Agha OM, Nicastri AD| title=An in-depth look at Krukenberg tumor: an overview. | journal=Arch Pathol Lab Med | year= 2006 | volume= 130 | issue= 11 | pages= 1725-30 | pmid=17076540 | doi=10.1043/1543-2165(2006)130[1725:AILAKT]2.0.CO;2 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17076540 }} </ref> | |||
*Stomach is the primary site in the majority of Krukenberg tumor cases (70%).<ref name="pmid17076540">{{cite journal| author=Al-Agha OM, Nicastri AD| title=An in-depth look at Krukenberg tumor: an overview. | journal=Arch Pathol Lab Med | year= 2006 | volume= 130 | issue= 11 | pages= 1725-30 | pmid=17076540 | doi=10.1043/1543-2165(2006)130[1725:AILAKT]2.0.CO;2 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17076540 }} </ref> | |||
*The pathogenesis of [disease name] is characterized by [feature1], [feature2], and [feature3]. | *The pathogenesis of [disease name] is characterized by [feature1], [feature2], and [feature3]. | ||
*The [gene name] gene/Mutation in [gene name] has been associated with the development of [disease name], involving the [molecular pathway] pathway. | *The [gene name] gene/Mutation in [gene name] has been associated with the development of [disease name], involving the [molecular pathway] pathway. | ||
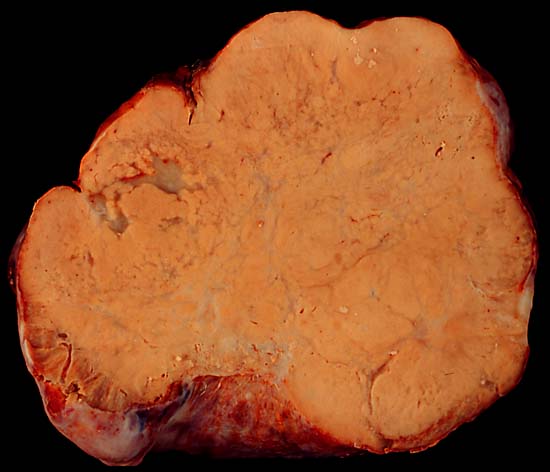
*On gross pathology, | *On gross pathology, asymmetrically enlarged ovaries with a bosselated contour, usually solid, yellow or white cross sectioned surfaces, and absence of adhesions or peritoneal deposits are characteristic findings of krukenberg tumors.<ref name="pmid17076540">{{cite journal| author=Al-Agha OM, Nicastri AD| title=An in-depth look at Krukenberg tumor: an overview. | journal=Arch Pathol Lab Med | year= 2006 | volume= 130 | issue= 11 | pages= 1725-30 | pmid=17076540 | doi=10.1043/1543-2165(2006)130[1725:AILAKT]2.0.CO;2 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17076540 }} </ref> | ||
*On microscopic histopathological analysis, | *On microscopic histopathological analysis, krukenberg tumors are characterized by the following features: | ||
**Tumor composed of two components: | |||
***Epithelial | |||
****Mucin-secreting signet ring cells with eccentric hyperchromatic nuclei | |||
****Cytoplasm may be eosinophilic and granular, pale and vacuolated, or a bull's eye (targetoid) appearance with a large vacuole with a central to paracentral eosinophilic body composed of a droplet of mucin | |||
****Signet ring cells may be single, clustered, nested, or arranged in tubules, acini, trabeculae, or cords | |||
***Stromal | |||
**** Plump and spindle-shaped cells with minimal cytologic atypia or mitotic activity | |||
****Focal or diffuse stromal edema which may form pseudo cysts | |||
****Desmoplastic reaction may be present | |||
*Stomach is the primary site in the majority of Krukenberg tumor cases (70%).<ref name="pmid17076540">{{cite journal| author=Al-Agha OM, Nicastri AD| title=An in-depth look at Krukenberg tumor: an overview. | journal=Arch Pathol Lab Med | year= 2006 | volume= 130 | issue= 11 | pages= 1725-30 | pmid=17076540 | doi=10.1043/1543-2165(2006)130[1725:AILAKT]2.0.CO;2 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17076540 }} </ref> | |||
==Causes== | ==Causes== | ||
* | * Krukenberg's tumor may be caused by either [cause1], [cause2], or [cause3]. | ||
* | * Krukenberg's tumor is caused by a mutation in the [gene1], [gene2], or [gene3] gene[s]. | ||
* There are no established causes for [disease name]. | * There are no established causes for [disease name]. | ||
==Differentiating [disease name] from other Diseases== | ==Differentiating [disease name] from other Diseases== | ||
* | *Krukenberg's tumor must be differentiated from other diseases that cause [clinical feature 1], [clinical feature 2], and [clinical feature 3], such as: | ||
:*[Differential dx1] | :*[Differential dx1] | ||
:*[Differential dx2] | :*[Differential dx2] | ||
| Line 56: | Line 70: | ||
*Patients of all age groups may develop [disease name]. | *Patients of all age groups may develop [disease name]. | ||
* | *Krukenberg's tumor is more commonly observed among patients aged [age range] years old. | ||
* | *Krukenberg's tumor is more commonly observed among [elderly patients/young patients/children]. | ||
===Gender=== | ===Gender=== | ||
* | *Krukenberg's tumor affects men and women equally. | ||
*[Gender 1] are more commonly affected with [disease name] than [gender 2]. | *[Gender 1] are more commonly affected with [disease name] than [gender 2]. | ||
| Line 68: | Line 82: | ||
*There is no racial predilection for [disease name]. | *There is no racial predilection for [disease name]. | ||
* | *Krukenberg's tumor usually affects individuals of the [race 1] race. | ||
*[Race 2] individuals are less likely to develop [disease name]. | *[Race 2] individuals are less likely to develop [disease name]. | ||
| Line 79: | Line 93: | ||
*If left untreated, [#%] of patients with [disease name] may progress to develop [manifestation 1], [manifestation 2], and [manifestation 3]. | *If left untreated, [#%] of patients with [disease name] may progress to develop [manifestation 1], [manifestation 2], and [manifestation 3]. | ||
*Common complications of [disease name] include [complication 1], [complication 2], and [complication 3]. | *Common complications of [disease name] include [complication 1], [complication 2], and [complication 3]. | ||
*Prognosis is generally | *Prognosis is generally poor, and the 5 year survival rate of patients with krukenberg tumor is lower in patients in with a preoperative serum CA 125 levels greater than 75 U/mL when compared with patients with CA 125 levels less than 75 U/mL.<ref name="pmid25830046">{{cite journal| author=Khan M, Bhatti RP, Mukherjee S, Ali AM, Gilman AD, Mirrakhimov AE et al.| title=A 26-year-old female with metastatic primary gastrointestinal malignancy presenting as menorrhagia. | journal=J Gastrointest Oncol | year= 2015 | volume= 6 | issue= 2 | pages= E21-5 | pmid=25830046 | doi=10.3978/j.issn.2078-6891.2014.080 | pmc=PMC4311099 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=25830046 }} </ref> | ||
== Diagnosis == | == Diagnosis == | ||
| Line 90: | Line 104: | ||
=== Symptoms === | === Symptoms === | ||
* | *Krukenberg's tumor is usually asymptomatic. | ||
*Symptoms of [disease name] may include the following: | *Symptoms of [disease name] may include the following: | ||
:*[symptom 1] | :*[symptom 1] | ||
| Line 124: | Line 138: | ||
=== Other Diagnostic Studies === | === Other Diagnostic Studies === | ||
* | ====Immunohistochemistry==== | ||
*Findings on | *Krukenberg's tumor may also be diagnosed using immunohistochemistry. | ||
*Findings on immunohistochemistry include: | |||
**Cytokeratins (AE1/AE3) positive | |||
**Epithelial membrane antigen positive | |||
**Vimentin negative | |||
**Inhibin negative | |||
====Serum CA-125=== | |||
*Serum concentrations of CA 125 may be helpful for:<ref name="pmid17076540">{{cite journal| author=Al-Agha OM, Nicastri AD| title=An in-depth look at Krukenberg tumor: an overview. | journal=Arch Pathol Lab Med | year= 2006 | volume= 130 | issue= 11 | pages= 1725-30 | pmid=17076540 | doi=10.1043/1543-2165(2006)130[1725:AILAKT]2.0.CO;2 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17076540 }} </ref> | |||
**Post-operative follow-up of patients for evaluation of complete resection of the tumor | |||
**Follow-up of patients with a history of primary adenocarcinomas (particularly gastrointestinal) for early detection of ovarian metastasis | |||
== Treatment == | == Treatment == | ||
| Line 145: | Line 169: | ||
*Effective measures for the primary prevention of [disease name] include [measure1], [measure2], and [measure3]. | *Effective measures for the primary prevention of [disease name] include [measure1], [measure2], and [measure3]. | ||
*Once diagnosed and successfully treated, patients with [disease name] are followed-up every [duration]. Follow-up testing includes [test 1], [test 2], and [test 3]. | *Once diagnosed and successfully treated, patients with [disease name] are followed-up every [duration]. Follow-up testing includes [test 1], [test 2], and [test 3]. | ||
==Case Studies== | ==Case Studies== | ||
Revision as of 00:45, 22 March 2016
| Krukenberg tumor | ||
 | ||
|---|---|---|
| Krukenberg tumor | ||
| ICD-10 | C56 | |
| ICD-9 | 183 | |
| ICD-O: | 8490/6 | |
| DiseasesDB | 30081 | |
| MeSH | C04.557.470.200.025.415.410 | |
|
WikiDoc Resources for Krukenberg tumor |
|
Articles |
|---|
|
Most recent articles on Krukenberg tumor Most cited articles on Krukenberg tumor |
|
Media |
|
Powerpoint slides on Krukenberg tumor |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Krukenberg tumor at Clinical Trials.gov Trial results on Krukenberg tumor Clinical Trials on Krukenberg tumor at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Krukenberg tumor NICE Guidance on Krukenberg tumor
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Krukenberg tumor Discussion groups on Krukenberg tumor Patient Handouts on Krukenberg tumor Directions to Hospitals Treating Krukenberg tumor Risk calculators and risk factors for Krukenberg tumor
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Krukenberg tumor |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Soujanya Thummathati, MBBS [2]
Synonyms and keywords: carcinoma mucocellulare; Synonym 2; Synonym 3
Overview
Krukenberg's tumor is a rare metastatic signet ring cell adenocarcinoma of the ovary.[1]
Historical Perspective
- Krukenberg's tumor was first described as a new type of primary ovarian malignancy by Friedrich Ernst Krukenberg (1871–1946), a German gynecologist and pathologist, in 1896 which was later confirmed to be of metastatic gastrointestinal tract origin.[1]
Classification
- Krukenberg's tumor may be classified according to [classification method] into [number] subtypes/groups:
- [group1]
- [group2]
- [group3]
- Other variants of [disease name] include [disease subtype 1], [disease subtype 2], and [disease subtype 3].
Pathophysiology
- The majority of Krukenberg’s tumors are bilateral.[1]
- Stomach is the primary site in the majority of Krukenberg tumor cases (70%).[1]
- The pathogenesis of [disease name] is characterized by [feature1], [feature2], and [feature3].
- The [gene name] gene/Mutation in [gene name] has been associated with the development of [disease name], involving the [molecular pathway] pathway.
- On gross pathology, asymmetrically enlarged ovaries with a bosselated contour, usually solid, yellow or white cross sectioned surfaces, and absence of adhesions or peritoneal deposits are characteristic findings of krukenberg tumors.[1]
- On microscopic histopathological analysis, krukenberg tumors are characterized by the following features:
**Tumor composed of two components:
- Epithelial
- Mucin-secreting signet ring cells with eccentric hyperchromatic nuclei
- Cytoplasm may be eosinophilic and granular, pale and vacuolated, or a bull's eye (targetoid) appearance with a large vacuole with a central to paracentral eosinophilic body composed of a droplet of mucin
- Signet ring cells may be single, clustered, nested, or arranged in tubules, acini, trabeculae, or cords
- Stromal
- Plump and spindle-shaped cells with minimal cytologic atypia or mitotic activity
- Focal or diffuse stromal edema which may form pseudo cysts
- Desmoplastic reaction may be present
- Epithelial
- Stomach is the primary site in the majority of Krukenberg tumor cases (70%).[1]
Causes
- Krukenberg's tumor may be caused by either [cause1], [cause2], or [cause3].
- Krukenberg's tumor is caused by a mutation in the [gene1], [gene2], or [gene3] gene[s].
- There are no established causes for [disease name].
Differentiating [disease name] from other Diseases
- Krukenberg's tumor must be differentiated from other diseases that cause [clinical feature 1], [clinical feature 2], and [clinical feature 3], such as:
- [Differential dx1]
- [Differential dx2]
- [Differential dx3]
Epidemiology and Demographics
- The prevalence of [disease name] is approximately [number or range] per 100,000 individuals worldwide.
- In [year], the incidence of [disease name] was estimated to be [number or range] cases per 100,000 individuals in [location].
Age
- Patients of all age groups may develop [disease name].
- Krukenberg's tumor is more commonly observed among patients aged [age range] years old.
- Krukenberg's tumor is more commonly observed among [elderly patients/young patients/children].
Gender
- Krukenberg's tumor affects men and women equally.
- [Gender 1] are more commonly affected with [disease name] than [gender 2].
- The [gender 1] to [Gender 2] ratio is approximately [number > 1] to 1.
Race
- There is no racial predilection for [disease name].
- Krukenberg's tumor usually affects individuals of the [race 1] race.
- [Race 2] individuals are less likely to develop [disease name].
Risk Factors
- Common risk factors in the development of [disease name] are [risk factor 1], [risk factor 2], [risk factor 3], and [risk factor 4].
Natural History, Complications and Prognosis
- The majority of patients with [disease name] remain asymptomatic for [duration/years].
- Early clinical features include [manifestation 1], [manifestation 2], and [manifestation 3].
- If left untreated, [#%] of patients with [disease name] may progress to develop [manifestation 1], [manifestation 2], and [manifestation 3].
- Common complications of [disease name] include [complication 1], [complication 2], and [complication 3].
- Prognosis is generally poor, and the 5 year survival rate of patients with krukenberg tumor is lower in patients in with a preoperative serum CA 125 levels greater than 75 U/mL when compared with patients with CA 125 levels less than 75 U/mL.[2]
Diagnosis
Diagnostic Criteria
- The diagnosis of [disease name] is made when at least [number] of the following [number] diagnostic criteria are met:
- [criterion 1]
- [criterion 2]
- [criterion 3]
- [criterion 4]
Symptoms
- Krukenberg's tumor is usually asymptomatic.
- Symptoms of [disease name] may include the following:
- [symptom 1]
- [symptom 2]
- [symptom 3]
- [symptom 4]
- [symptom 5]
- [symptom 6]
Physical Examination
- Patients with [disease name] usually appear [general appearance].
- Physical examination may be remarkable for:
- [finding 1]
- [finding 2]
- [finding 3]
- [finding 4]
- [finding 5]
- [finding 6]
Laboratory Findings
- There are no specific laboratory findings associated with [disease name].
- A [positive/negative] [test name] is diagnostic of [disease name].
- An [elevated/reduced] concentration of [serum/blood/urinary/CSF/other] [lab test] is diagnostic of [disease name].
- Other laboratory findings consistent with the diagnosis of [disease name] include [abnormal test 1], [abnormal test 2], and [abnormal test 3].
Imaging Findings
- There are no [imaging study] findings associated with [disease name].
- [Imaging study 1] is the imaging modality of choice for [disease name].
- On [imaging study 1], [disease name] is characterized by [finding 1], [finding 2], and [finding 3].
- [Imaging study 2] may demonstrate [finding 1], [finding 2], and [finding 3].
Other Diagnostic Studies
Immunohistochemistry
- Krukenberg's tumor may also be diagnosed using immunohistochemistry.
- Findings on immunohistochemistry include:
- Cytokeratins (AE1/AE3) positive
- Epithelial membrane antigen positive
- Vimentin negative
- Inhibin negative
=Serum CA-125
- Serum concentrations of CA 125 may be helpful for:[1]
- Post-operative follow-up of patients for evaluation of complete resection of the tumor
- Follow-up of patients with a history of primary adenocarcinomas (particularly gastrointestinal) for early detection of ovarian metastasis
Treatment
Medical Therapy
- There is no treatment for [disease name]; the mainstay of therapy is supportive care.
- The mainstay of therapy for [disease name] is [medical therapy 1] and [medical therapy 2].
- [Medical therapy 1] acts by [mechanism of action1].
- Response to [medical therapy 1] can be monitored with [test/physical finding/imaging] every [frequency/duration].
Surgery
- Surgery is the mainstay of therapy for [disease name].
- [Surgical procedure] in conjunction with [chemotherapy/radiation] is the most common approach to the treatment of [disease name].
- [Surgical procedure] can only be performed for patients with [disease stage] [disease name].
Prevention
- There are no primary preventive measures available for [disease name].
- Effective measures for the primary prevention of [disease name] include [measure1], [measure2], and [measure3].
- Once diagnosed and successfully treated, patients with [disease name] are followed-up every [duration]. Follow-up testing includes [test 1], [test 2], and [test 3].
Case Studies
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Al-Agha OM, Nicastri AD (2006). "An in-depth look at Krukenberg tumor: an overview". Arch Pathol Lab Med. 130 (11): 1725–30. doi:10.1043/1543-2165(2006)130[1725:AILAKT]2.0.CO;2. PMID 17076540.
- ↑ Khan M, Bhatti RP, Mukherjee S, Ali AM, Gilman AD, Mirrakhimov AE; et al. (2015). "A 26-year-old female with metastatic primary gastrointestinal malignancy presenting as menorrhagia". J Gastrointest Oncol. 6 (2): E21–5. doi:10.3978/j.issn.2078-6891.2014.080. PMC 4311099. PMID 25830046.