Klebsiella pneumoniae
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
| Klebsiella pneumoniae | ||
| ICD-10 | B96.1, G00.8, J15.0, P23.6 | |
|---|---|---|
| ICD-9 | 041.3, 320.82, 482.0 | |
| DiseasesDB | 7181 | |
| eMedicine | med/1237 | |
| MeSH | C01.252.400.310.503 | |
| Klebsiella pneumoniae | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
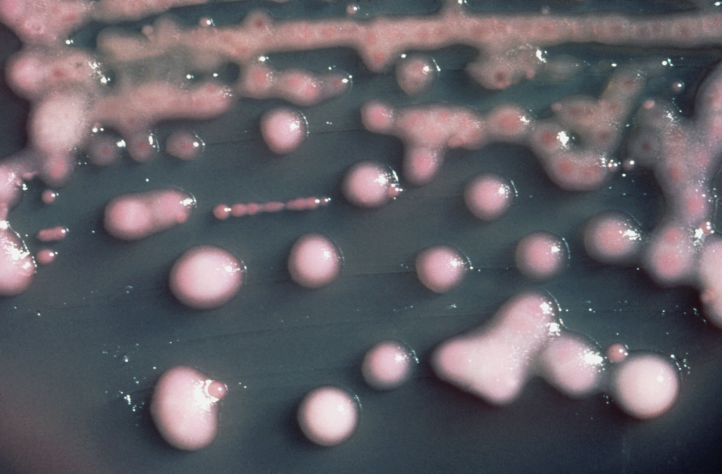 K. pneumoniae on a MacConkey agar plate.
| ||||||||||||||
| Scientific classification | ||||||||||||||
| ||||||||||||||
| Binomial name | ||||||||||||||
| Klebsiella pneumoniae (Schroeter 1886) Trevisan 1887 |
Klebsiella pneumoniae is a Gram-negative, non-motile, encapsulated, lactose fermenting, facultative anaerobic, rod shaped bacterium found in the normal flora of the mouth, skin, and intestines.[1] It is clinically the most important member of the Klebsiella genus of Enterobacteriaceae; it is closely related to K. oxytoca from which it is distinguished by being indole-negative and by its ability to grow on both melezitose and 3-hydroxybutyrate. It naturally occurs in the soil and about 30% of strains can fix nitrogen in anaerobic condition.[2] As a free-living diazotroph, its nitrogen fixation system has been much studied.
New antibiotic resistant strains of K. pneumoniae are appearing, and it is increasingly found as a nosocomial infection.
History
The Danish scientist Hans Christian Gram (1853–1938), developed the technique now known as Gram staining in 1884 to discriminate between K. pneumoniae and Streptococcus pneumoniae.
Klebsiella was named after the German bacteriologist Edwin Klebs (1834–1913).
Community-acquired pneumonia caused by Klebsiella pneumoniae may be called Friedländer's Pneumonia, after Carl Friedländer.
Pathogenesis
K. pneumoniae can cause bacterial pneumonia, typically due to aspiration by alcoholics, though it is more commonly implicated in hospital-acquired urinary tract and wound infections, particularly in immunocompromised individuals. Klebsiella ranks second to E. coli for urinary tract infections in older persons. It is also an opportunistic pathogen for patients with chronic pulmonary disease, enteric pathogenicity, nasal mucosa atrophy, and rhinoscleroma. Feces are the most significant source of patient infection, followed by contact with contaminated instruments.
Members of the Klebsiella genus typically express 2 types of antigens on their cell surface. The first, O antigen, is a lipopolysaccharide of which 77 varieties exist. The second is K agent, a capsular polysaccharide with 9 varieties. Both contribute to pathogenicity and form the basis for subtyping.
Research conducted at King's College, London has implicated molecular mimicry between HLA-B27 and two molecules in Klebsiella microbes as the cause of ankylosing spondylitis.[3] As a general rule, Klebsiella infections tend to occur in people with a weakened immune system from improper diet. Many of these infections are obtained when a person is in the hospital for some other reason. The most common infection caused by Klebsiella bacteria outside the hospital is pneumonia.
Klebsiella pneumonia tends to affect people with underlying diseases, such as alcoholism, diabetes and chronic lung disease.
Treatment
Klebsiella possesses a chromosomal class A beta-lactamase giving it inherent resistance to ampicillin. Many strains have acquired an extended-spectrum beta-lactamase with additional resistance to carbenicillin, ampicillin, quinolones, and increasingly to ceftazidime. The bacteria remain largely susceptible to aminoglycosides and cephalosporins. Varying degrees of inhibition of the beta-lactamase with clavulanic acid have been reported. Infections due to multidrug-resistant Gram-negative pathogens in the ICU have invoked the re-emergence of colistin, an antibiotic that had rarely been used for decades. However, colistin-resistant strains of K. pneumoniae have been reported in Greek ICUs.4
Antimicrobial regimen
- Klebsiella pneumoniae[4]
- 1. Severe, nosocomial infections
- 1.1 Non-ESBLs in pneumonia, sepsis, complicated UTI, or intra-abdominal infections
- Preferred regimen (1): Cefepime 2 g IV q8h
- Preferred regimen (2): Ceftazidime 2 g IV q8h
- Preferred regimen (3): Imipenem 500 mg IV q6h
- Preferred regimen (4): Meropenem 1 g IV q8h
- Preferred regimen (5): Piperacillin-tazobactam 4.5 g IV q6h AND Aminoglycoside
- Alternative regimen (1): Ceftriaxone 1 g IV q24h AND Metronidazole 500 mg IV q6h or 1 g IV q12h
- Alternative regimen (2): Moxifloxacin 400 mg IV/PO q24h
- 1.2 ESBLs in pneumonia, sepsis, complicated UTI, or intra-abdominal infections
- Preferred regimen (1): Imipenem 500 mg IV q6h
- Preferred regimen (2): Meropenem 1 g IV q8h
- Preferred regimen (3): Ertapenem 1 g IV q24h
- Preferred regimen (4): Doripenem 500 mg IV q8h
- Note: In ESBLs, inconsistent activity is seen with aminoglycosides, fluoroquinolones, and piperacillin-tazobactam. Avoid cephalosporins.
References
- ↑ Ryan KJ; Ray CG (editors) (2004). Sherris Medical Microbiology (4th ed. ed.). McGraw Hill. ISBN 0838585299.
- ↑ Postgate J (1998). Nitrogen fixation, 3rd ed. Cambridge University Press.
- ↑ Rashid T, Ebringer A (2006). "Ankylosing spondylitis is linked to Klebsiella-the evidence (Epub ahead of print)". Clin Rheumatol. PMID 17186116.
- ↑ Bartlett, John (2012). Johns Hopkins ABX guide : diagnosis and treatment of infectious diseases. Burlington, MA: Jones and Bartlett Learning. ISBN 978-1449625580.
4. Antoniadou, A. et al. (2006). Colistin-resistant isolates of Klebsiella pneumoniae emerging in intensive care unit patients: first report of a multiclonal cluster. The Journal of Antimicrobial Chemotherapy. Retrieved on April 28, 2007 from http://jac.oxfordjournals.org/cgi/content/abstract/dkl562v1
External links
| Wikimedia Commons has media related to Klebsiella pneumoniae. |
- Diseases, symptoms, diagnosis of K. pneumoniae (University of Florida)
- Virtual museum of bacteria page on K. pneumoniae
- What're the complications of pneumonia? (health-cares.net)
- Klebsiella Infection (emedicine.com)
- Klebsiella Genome Projects from Genomes OnLine Database