Ischemic stroke pathophysiology: Difference between revisions
No edit summary |
No edit summary |
||
| Line 4: | Line 4: | ||
==Pathophysiology== | ==Pathophysiology== | ||
Ischemic stroke | ==Pathophysiology== | ||
===Ischemic Stroke=== | |||
==== Thrombotic Stroke ==== | |||
In thrombotic stroke, a [[thrombus]]-forming process develops in the affected artery. The thrombus — a built-up clot — gradually narrows the lumen of the artery and impedes [[blood flow]] to distal tissue. These clots usually form around [[atherosclerosis|atherosclerotic]] plaques. Since blockage of the artery is gradual, onset of symptomatic thrombotic strokes is slower. A thrombus itself (even if non-occluding) can lead to an embolic stroke (see below) if the thrombus breaks off—at which point it is then called an "embolus." | |||
====Embolic Stroke==== | |||
Embolic stroke refers to the blockage of arterial access to a part of the brain by an [[embolus]]—a traveling particle or debris in the arterial bloodstream originating from elsewhere. An embolus is most frequently a blood clot, but it can also be a plaque broken off from an [[atherosclerosis|atherosclerotic]] blood vessel or a number of other substances including fat (e.g., from [[bone marrow]] in a [[Bone fracture|broken bone]]), air, and even [[cancer]]ous cells. Another cause is bacterial emboli released in infectious [[endocarditis]]. | |||
Because an embolus arises from elsewhere, local therapy only solves the problem temporarily. Thus, the source of the embolus must be identified. Because the embolic blockage is sudden in onset, symptoms usually are maximal at start. Also, symptoms may be transient as the embolus lyses and moves to a different location or dissipates altogether. | |||
Embolic stroke can be divided into four categories: | |||
* Those with known cardiac source | |||
* Those with potential cardiac or aortic source (from [[echocardiogram|transthoracic]] or [[transesophageal echocardiogram]]) | |||
* Those with an arterial source | |||
* Those with unknown source | |||
====Systemic Hypoperfusion (Watershed Stroke)==== | |||
Systemic hypoperfusion is the reduction of blood flow to all parts of the body. It is most commonly due to [[Heart-lung machine|cardiac pump]] failure from [[cardiac arrest]] or arrhythmias, or from reduced [[cardiac output]] as a result of [[myocardial infarction]], [[pulmonary embolism]], [[pericardial effusion]], or bleeding. [[Hypoxia (medical)|Hypoxemia]] (low blood oxygen content) may precipitate the hypoperfusion. Because the reduction in blood flow is global, all parts of the brain may be affected, especially "watershed" areas --- border zone regions supplied by the major cerebral arteries. Blood flow to these areas does not necessarily stop, but instead it may lessen to the point where [[brain damage]] can occur. This phenomenon is also referred to as "last meadow" to point to the fact that in irrigation the last meadow receives the least amount of water. | |||
Ischemic stroke occurs due to a loss of blood supply to part of the brain, initiating the [[Ischemic cascade]]. Brain tissue ceases to function if deprived of oxygen for more than 60 to 90 seconds and after a few hours will suffer irreversible injury possibly leading to death of the tissue, i.e., [[infarction]]. [[Atherosclerosis]] may disrupt the blood supply by narrowing the lumen of blood vessels leading to a reduction of blood flow, by causing the formation of blood clots within the vessel, or by releasing showers of small [[emboli]] through the disintegration of atherosclerotic plaques. Embolic infarction occurs when emboli formed elsewhere in the circulatory system, typically in the heart as a consequence of [[atrial fibrillation]], or in the carotid arteries. These break off, enter the cerebral circulation, then lodge in and occlude brain blood vessels. | |||
Due to [[Anastomosis|collateral circulation]], within the region of brain tissue affected by ischemia there is a spectrum of severity. Thus, part of the tissue may immediately die while other parts may only be injured and could potentially recover. The ischemia area where tissue might recover is referred to as the ''ischemic penumbra''. | |||
As oxygen or glucose becomes depleted in ischemic brain tissue, the production of [[high energy phosphate]] compounds such as adenine triphosphate (ATP) fails leading to failure of energy dependent processes (such as ion pumping) necessary for tissue cell survival. This sets off a series of interrelated events that result in cellular injury and death. A major cause of neuronal injury is release of the excitatory neurotransmitter glutamate. The concentration of glutamate outside the cells of the nervous system is normally kept low by so-called uptake carriers, which are powered by the concentration gradients of ions (mainly Na<sup>+</sup>) across the cell membrane. However, stroke cuts off the supply of oxygen and glucose which powers the ion pumps maintaining these gradients. As a result the transmembrane ion gradients run down, and glutamate transporters reverse their direction, releasing glutamate into the extracellular space. Glutamate acts on receptors in nerve cells (especially NMDA receptors), producing an influx of calcium which activates enzymes that digest the cells' proteins, lipids and nuclear material. Calcium influx can also lead to the failure of [[mitochondria]], which can lead further toward energy depletion and may trigger cell death due to [[apoptosis]]. | |||
Ischaemia also induces production of [[Radical (chemistry)|oxygen free radicals]] and other [[reactive oxygen species]]. These react with and damage a number of cellular and extracellular elements. Damage to the blood vessel lining or [[endothelium]] is particularly important. In fact, many antioxidant neuroprotectants such as [[uric acid]] and [[NXY-059]] work at the level of the [[endothelium]] and not in the brain ''per se''. Free radicals also directly initiate elements of the [[apoptosis]] cascade by means of [[redox signaling]]. | |||
These processes are the same for any type of ischemic tissue and are referred to collectively as the ''[[ischemic cascade]]''. However, brain tissue is especially vulnerable to ischemia since it has little respiratory reserve and is completely dependent on [[aerobic metabolism]], unlike most other organs. | |||
Brain tissue survival can be improved to some extent if one or more of these processes is inhibited. Drugs that scavenge [[Reactive oxygen species]], inhibit [[apoptosis]], or inhibit excitotoxic neurotransmitters, for example, have been shown experimentally to reduce tissue injury due to ischemia. Agents that work in this way are referred to as being ''neuroprotective''. Until recently, human [[clinical trial]]s with neuroprotective agents have failed, with the probable exception of deep barbiturate coma. However, more recently [[NXY-059]], the disulfonyl derivative of the radical-scavenging spintrap phenylbutylnitrone, is [http://content.nejm.org/cgi/content/short/354/6/588 reported] be neuroprotective in stroke. This agent appears to work at the level of the blood vessel lining or [[endothelium]]. Unfortunately, after producing favorable results in one large-scale clinical trial, a second trial failed to show favorable results. | |||
In addition to injurious effects on brain cells, ischemia and infarction can result in loss of structural integrity of brain tissue and blood vessels, partly through the release of matrix metalloproteases, which are zinc- and calcium-dependent enzymes that break down collagen, [[Hyaluronan|hyaluronic acid]], and other elements of [[connective tissue]]. Other proteases also contribute to this process. The loss of vascular structural integrity results in a breakdown of the protective [[blood brain barrier]] that contributes to [[cerebral edema]], which can cause secondary progression of the brain injury. | |||
As is the case with any type of [[Traumatic brain injury|brain injury]], the [[immune system]] is activated by cerebral infarction and may under some circumstances exacerbate the injury caused by the infarction. Inhibition of the [[Inflammation|inflammatory response]] has been shown experimentally to reduce tissue injury due to cerebral infarction, but this has not proved out in clinical studies. | |||
===Hemorrhagic Stroke=== | |||
A hemorrhagic stroke, or [[cerebral hemorrhage]], is a form of stroke that occurs when a blood vessel in the brain ruptures or bleeds. Like ischemic strokes, hemorrhagic strokes interrupt the brain's blood supply because the bleeding vessel can no longer carry the blood to its target tissue. In addition, blood irritates brain tissue, disrupting the delicate chemical balance, and, if the bleeding continues, it can cause increased intracranial pressure which physically impinges on brain tissue and restricts blood flow into the brain. In this respect, hemorrhagic strokes are more dangerous than their more common counterpart, ischemic strokes. There are two types of hemorrhagic stroke: intracerebral hemorrhage, and subarachnoid hemorrhage. Amphetamine abuse quintuples, and cocaine abuse doubles, the risk of hemorrhagic strokes. | |||
====Intracerebral Hemorrhage==== | |||
{{main|intracerebral hemorrhage}} | |||
Intracerebral hemorrhage (ICH) is bleeding directly into the brain tissue, forming a gradually enlarging [[hematoma]] (pooling of blood). The hematoma enlarges until pressure from surrounding tissue limits its growth, or until it decompresses by emptying into the [[ventricular system]], [[cerebrospinal fluid|CSF]] or the [[pia]]l surface. A third of intracerebral bleed is into the brain's ventricles. ICH has a [[mortality rate]] of 44 percent after 30 days, higher than ischemic stroke or even the very deadly subarachnoid hemorrhage.<ref name="caplan">{{cite journal | author= Caplan LR | title= Intracerebral hemorrhage | journal= Lancet | year=1992 | pages=656-8 | volume=339 | issue=8794 | id=PMID 1347346}}</ref> | |||
==Gross Pathology== | |||
===Ischemic Stroke=== | |||
{| align="center" | |||
|-valign="top" | |||
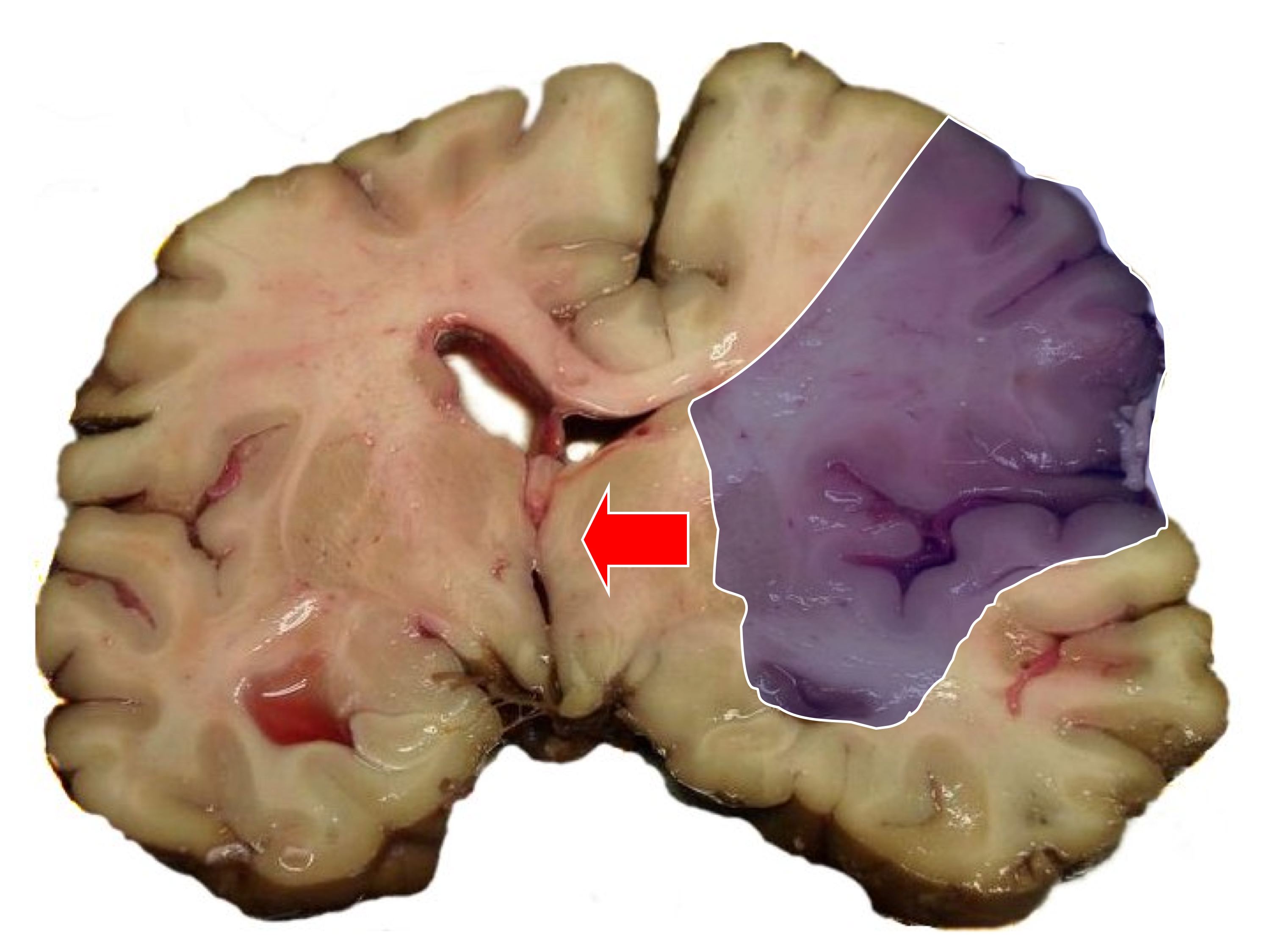
| [[Image:MCA-Stroke-Brain-Human-2.JPG|thumb|Autopsy of brain showing [[middle cerebral artery]] territory]] | |||
| [[Image:MCA-Stroke-Brain-Humn-2A.jpg|thumb|Same image; infarct area (blue shading) and midline shift]] | |||
|} | |||
==References== | ==References== | ||
{{reflist|2}} | {{reflist|2}} | ||
Revision as of 15:34, 6 February 2013
|
Stroke Main page | |
|
Diagnosis | |
|---|---|
|
Treatment | |
|
Case Studies | |
|
Ischemic stroke pathophysiology On the Web | |
|
American Roentgen Ray Society Images of Ischemic stroke pathophysiology | |
|
Risk calculators and risk factors for Ischemic stroke pathophysiology | |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Pathophysiology
Pathophysiology
Ischemic Stroke
Thrombotic Stroke
In thrombotic stroke, a thrombus-forming process develops in the affected artery. The thrombus — a built-up clot — gradually narrows the lumen of the artery and impedes blood flow to distal tissue. These clots usually form around atherosclerotic plaques. Since blockage of the artery is gradual, onset of symptomatic thrombotic strokes is slower. A thrombus itself (even if non-occluding) can lead to an embolic stroke (see below) if the thrombus breaks off—at which point it is then called an "embolus."
Embolic Stroke
Embolic stroke refers to the blockage of arterial access to a part of the brain by an embolus—a traveling particle or debris in the arterial bloodstream originating from elsewhere. An embolus is most frequently a blood clot, but it can also be a plaque broken off from an atherosclerotic blood vessel or a number of other substances including fat (e.g., from bone marrow in a broken bone), air, and even cancerous cells. Another cause is bacterial emboli released in infectious endocarditis.
Because an embolus arises from elsewhere, local therapy only solves the problem temporarily. Thus, the source of the embolus must be identified. Because the embolic blockage is sudden in onset, symptoms usually are maximal at start. Also, symptoms may be transient as the embolus lyses and moves to a different location or dissipates altogether. Embolic stroke can be divided into four categories:
- Those with known cardiac source
- Those with potential cardiac or aortic source (from transthoracic or transesophageal echocardiogram)
- Those with an arterial source
- Those with unknown source
Systemic Hypoperfusion (Watershed Stroke)
Systemic hypoperfusion is the reduction of blood flow to all parts of the body. It is most commonly due to cardiac pump failure from cardiac arrest or arrhythmias, or from reduced cardiac output as a result of myocardial infarction, pulmonary embolism, pericardial effusion, or bleeding. Hypoxemia (low blood oxygen content) may precipitate the hypoperfusion. Because the reduction in blood flow is global, all parts of the brain may be affected, especially "watershed" areas --- border zone regions supplied by the major cerebral arteries. Blood flow to these areas does not necessarily stop, but instead it may lessen to the point where brain damage can occur. This phenomenon is also referred to as "last meadow" to point to the fact that in irrigation the last meadow receives the least amount of water.
Ischemic stroke occurs due to a loss of blood supply to part of the brain, initiating the Ischemic cascade. Brain tissue ceases to function if deprived of oxygen for more than 60 to 90 seconds and after a few hours will suffer irreversible injury possibly leading to death of the tissue, i.e., infarction. Atherosclerosis may disrupt the blood supply by narrowing the lumen of blood vessels leading to a reduction of blood flow, by causing the formation of blood clots within the vessel, or by releasing showers of small emboli through the disintegration of atherosclerotic plaques. Embolic infarction occurs when emboli formed elsewhere in the circulatory system, typically in the heart as a consequence of atrial fibrillation, or in the carotid arteries. These break off, enter the cerebral circulation, then lodge in and occlude brain blood vessels.
Due to collateral circulation, within the region of brain tissue affected by ischemia there is a spectrum of severity. Thus, part of the tissue may immediately die while other parts may only be injured and could potentially recover. The ischemia area where tissue might recover is referred to as the ischemic penumbra.
As oxygen or glucose becomes depleted in ischemic brain tissue, the production of high energy phosphate compounds such as adenine triphosphate (ATP) fails leading to failure of energy dependent processes (such as ion pumping) necessary for tissue cell survival. This sets off a series of interrelated events that result in cellular injury and death. A major cause of neuronal injury is release of the excitatory neurotransmitter glutamate. The concentration of glutamate outside the cells of the nervous system is normally kept low by so-called uptake carriers, which are powered by the concentration gradients of ions (mainly Na+) across the cell membrane. However, stroke cuts off the supply of oxygen and glucose which powers the ion pumps maintaining these gradients. As a result the transmembrane ion gradients run down, and glutamate transporters reverse their direction, releasing glutamate into the extracellular space. Glutamate acts on receptors in nerve cells (especially NMDA receptors), producing an influx of calcium which activates enzymes that digest the cells' proteins, lipids and nuclear material. Calcium influx can also lead to the failure of mitochondria, which can lead further toward energy depletion and may trigger cell death due to apoptosis.
Ischaemia also induces production of oxygen free radicals and other reactive oxygen species. These react with and damage a number of cellular and extracellular elements. Damage to the blood vessel lining or endothelium is particularly important. In fact, many antioxidant neuroprotectants such as uric acid and NXY-059 work at the level of the endothelium and not in the brain per se. Free radicals also directly initiate elements of the apoptosis cascade by means of redox signaling.
These processes are the same for any type of ischemic tissue and are referred to collectively as the ischemic cascade. However, brain tissue is especially vulnerable to ischemia since it has little respiratory reserve and is completely dependent on aerobic metabolism, unlike most other organs.
Brain tissue survival can be improved to some extent if one or more of these processes is inhibited. Drugs that scavenge Reactive oxygen species, inhibit apoptosis, or inhibit excitotoxic neurotransmitters, for example, have been shown experimentally to reduce tissue injury due to ischemia. Agents that work in this way are referred to as being neuroprotective. Until recently, human clinical trials with neuroprotective agents have failed, with the probable exception of deep barbiturate coma. However, more recently NXY-059, the disulfonyl derivative of the radical-scavenging spintrap phenylbutylnitrone, is reported be neuroprotective in stroke. This agent appears to work at the level of the blood vessel lining or endothelium. Unfortunately, after producing favorable results in one large-scale clinical trial, a second trial failed to show favorable results.
In addition to injurious effects on brain cells, ischemia and infarction can result in loss of structural integrity of brain tissue and blood vessels, partly through the release of matrix metalloproteases, which are zinc- and calcium-dependent enzymes that break down collagen, hyaluronic acid, and other elements of connective tissue. Other proteases also contribute to this process. The loss of vascular structural integrity results in a breakdown of the protective blood brain barrier that contributes to cerebral edema, which can cause secondary progression of the brain injury.
As is the case with any type of brain injury, the immune system is activated by cerebral infarction and may under some circumstances exacerbate the injury caused by the infarction. Inhibition of the inflammatory response has been shown experimentally to reduce tissue injury due to cerebral infarction, but this has not proved out in clinical studies.
Hemorrhagic Stroke
A hemorrhagic stroke, or cerebral hemorrhage, is a form of stroke that occurs when a blood vessel in the brain ruptures or bleeds. Like ischemic strokes, hemorrhagic strokes interrupt the brain's blood supply because the bleeding vessel can no longer carry the blood to its target tissue. In addition, blood irritates brain tissue, disrupting the delicate chemical balance, and, if the bleeding continues, it can cause increased intracranial pressure which physically impinges on brain tissue and restricts blood flow into the brain. In this respect, hemorrhagic strokes are more dangerous than their more common counterpart, ischemic strokes. There are two types of hemorrhagic stroke: intracerebral hemorrhage, and subarachnoid hemorrhage. Amphetamine abuse quintuples, and cocaine abuse doubles, the risk of hemorrhagic strokes.
Intracerebral Hemorrhage
Intracerebral hemorrhage (ICH) is bleeding directly into the brain tissue, forming a gradually enlarging hematoma (pooling of blood). The hematoma enlarges until pressure from surrounding tissue limits its growth, or until it decompresses by emptying into the ventricular system, CSF or the pial surface. A third of intracerebral bleed is into the brain's ventricles. ICH has a mortality rate of 44 percent after 30 days, higher than ischemic stroke or even the very deadly subarachnoid hemorrhage.[1]
Gross Pathology
Ischemic Stroke
 |
 |
References
- ↑ Caplan LR (1992). "Intracerebral hemorrhage". Lancet. 339 (8794): 656–8. PMID 1347346.