Insulinoma pathophysiology: Difference between revisions
Jump to navigation
Jump to search
| Line 15: | Line 15: | ||
*Insulinoma is a rare benign [[pancreatic neuroendocrine tumor]] that arises from β islet cells, which are cells that are normally involved in the production of [[insulin]]. Few insulinomas can also produce other hormones such as [[Serotonin]], [[gastrin]], [[ACTH]], [[glucagon]], and [[somatostatin]] <ref name="AlJadir2015">{{cite journal|last1=AlJadir|first1=Saadi|title=Insulinoma: Literature’s Review (Part 1)|journal=Endocrinology&Metabolism International Journal|volume=2|issue=3|year=2015|issn=24730815|doi=10.15406/emij.2015.02.00025}}</ref> | *Insulinoma is a rare benign [[pancreatic neuroendocrine tumor]] that arises from β islet cells, which are cells that are normally involved in the production of [[insulin]]. Few insulinomas can also produce other hormones such as [[Serotonin]], [[gastrin]], [[ACTH]], [[glucagon]], and [[somatostatin]] <ref name="AlJadir2015">{{cite journal|last1=AlJadir|first1=Saadi|title=Insulinoma: Literature’s Review (Part 1)|journal=Endocrinology&Metabolism International Journal|volume=2|issue=3|year=2015|issn=24730815|doi=10.15406/emij.2015.02.00025}}</ref> | ||
* They are usually small(90%), sporadic(90%), solitary(90%) and benign(90%) tumors. | * They are usually small(90%), sporadic(90%), [[solitary]](90%) and [[benign]](90%) tumors. | ||
* It usually occurs sporadically but 10% are found to be associated with [[MEN 1]] syndrome.<ref name="pmid18672144">{{cite journal| author=Callender GG, Rich TA, Perrier ND| title=Multiple endocrine neoplasia syndromes. | journal=Surg Clin North Am | year= 2008 | volume= 88 | issue= 4 | pages= 863-95, viii | pmid=18672144 | doi=10.1016/j.suc.2008.05.001 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18672144 }} </ref>Those associated with the MEN1 syndrome are usually malignant and higher recurrence rate(21% at 10 and 20 years) than in those without [[MEN 1]] (5% at 10 and 7% at 20 years). <ref name="pmid1677058">{{cite journal| author=Service FJ, McMahon MM, O'Brien PC, Ballard DJ| title=Functioning insulinoma--incidence, recurrence, and long-term survival of patients: a 60-year study. | journal=Mayo Clin Proc | year= 1991 | volume= 66 | issue= 7 | pages= 711-9 | pmid=1677058 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=1677058 }} </ref>. | * It usually occurs sporadically but 10% are found to be associated with [[MEN 1]] syndrome.<ref name="pmid18672144">{{cite journal| author=Callender GG, Rich TA, Perrier ND| title=Multiple endocrine neoplasia syndromes. | journal=Surg Clin North Am | year= 2008 | volume= 88 | issue= 4 | pages= 863-95, viii | pmid=18672144 | doi=10.1016/j.suc.2008.05.001 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18672144 }} </ref>Those associated with the MEN1 syndrome are usually malignant and higher recurrence rate(21% at 10 and 20 years) than in those without [[MEN 1]] (5% at 10 and 7% at 20 years). <ref name="pmid1677058">{{cite journal| author=Service FJ, McMahon MM, O'Brien PC, Ballard DJ| title=Functioning insulinoma--incidence, recurrence, and long-term survival of patients: a 60-year study. | journal=Mayo Clin Proc | year= 1991 | volume= 66 | issue= 7 | pages= 711-9 | pmid=1677058 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=1677058 }} </ref>. | ||
*It is thought that insulinoma is mediated by mTOR/P70S6K signaling pathway. Inhibitors of mTOR([[rapamycin]]) or dual PI3K/mTOR(NVP-BEZ2235)thus have become new drugs for treating insulinoma. An oral mTOR inhibitor, [[Everolimus]], make better glycemic | *It is thought that insulinoma is mediated by mTOR/P70S6K signaling pathway. Inhibitors of mTOR([[rapamycin]]) or dual PI3K/mTOR(NVP-BEZ2235)thus have become new drugs for treating insulinoma. An oral mTOR inhibitor, [[Everolimus]], make better [[Glycemic control|glycemic contro]]<nowiki/>l in people having insulinoma.<ref name="pmid19129539">{{cite journal| author=Kulke MH, Bergsland EK, Yao JC| title=Glycemic control in patients with insulinoma treated with everolimus. | journal=N Engl J Med | year= 2009 | volume= 360 | issue= 2 | pages= 195-7 | pmid=19129539 | doi=10.1056/NEJMc0806740 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19129539 }} </ref> The pathway will give more possibilities for medical treatment.<ref name="pmid22711648">{{cite journal| author=Zhan HX, Cong L, Zhao YP, Zhang TP, Chen G, Zhou L et al.| title=Activated mTOR/P70S6K signaling pathway is involved in insulinoma tumorigenesis. | journal=J Surg Oncol | year= 2012 | volume= 106 | issue= 8 | pages= 972-80 | pmid=22711648 | doi=10.1002/jso.23176 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=22711648 }} </ref> | ||
*[[Mitochondria]] play a key role in glucose and insulin coupling to assure insulin secretion after glucose stimulation in pancreatic β cells. Coupling is impaired due to abnormal mitochondrial function in β cells causes the death of the cell.<ref name="pmid22766318">{{cite journal| author=Supale S, Li N, Brun T, Maechler P| title=Mitochondrial dysfunction in pancreatic β cells. | journal=Trends Endocrinol Metab | year= 2012 | volume= 23 | issue= 9 | pages= 477-87 | pmid=22766318 | doi=10.1016/j.tem.2012.06.002 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=22766318 }} </ref>YY1 regulates this mitochondrial function.<ref name="pmid18046414">{{cite journal| author=Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P| title=mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. | journal=Nature | year= 2007 | volume= 450 | issue= 7170 | pages= 736-40 | pmid=18046414 | doi=10.1038/nature06322 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18046414 }} </ref>T372R mutation increase the [[transcription]] of YY1.<ref name="CaoGao2013">{{cite journal|last1=Cao|first1=Yanan|last2=Gao|first2=Zhibo|last3=Li|first3=Lin|last4=Jiang|first4=Xiuli|last5=Shan|first5=Aijing|last6=Cai|first6=Jie|last7=Peng|first7=Ying|last8=Li|first8=Yanli|last9=Jiang|first9=Xiaohua|last10=Huang|first10=Xuanlin|last11=Wang|first11=Jiaqian|last12=Wei|first12=Qing|last13=Qin|first13=Guijun|last14=Zhao|first14=Jiajun|last15=Jin|first15=Xiaolong|last16=Liu|first16=Li|last17=Li|first17=Yingrui|last18=Wang|first18=Weiqing|last19=Wang|first19=Jun|last20=Ning|first20=Guang|title=Whole exome sequencing of insulinoma reveals recurrent T372R mutations in YY1|journal=Nature Communications|volume=4|year=2013|issn=2041-1723|doi=10.1038/ncomms3810}}</ref>The understanding of role and functions of YY1 in β cells in near future might prove to be therapeutic potentials. | *[[Mitochondria]] play a key role in glucose and insulin coupling to assure insulin secretion after glucose stimulation in pancreatic β cells. Coupling is impaired due to abnormal mitochondrial function in β cells causes the death of the cell.<ref name="pmid22766318">{{cite journal| author=Supale S, Li N, Brun T, Maechler P| title=Mitochondrial dysfunction in pancreatic β cells. | journal=Trends Endocrinol Metab | year= 2012 | volume= 23 | issue= 9 | pages= 477-87 | pmid=22766318 | doi=10.1016/j.tem.2012.06.002 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=22766318 }} </ref>YY1 regulates this mitochondrial function.<ref name="pmid18046414">{{cite journal| author=Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P| title=mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. | journal=Nature | year= 2007 | volume= 450 | issue= 7170 | pages= 736-40 | pmid=18046414 | doi=10.1038/nature06322 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18046414 }} </ref>T372R mutation increase the [[transcription]] of YY1.<ref name="CaoGao2013">{{cite journal|last1=Cao|first1=Yanan|last2=Gao|first2=Zhibo|last3=Li|first3=Lin|last4=Jiang|first4=Xiuli|last5=Shan|first5=Aijing|last6=Cai|first6=Jie|last7=Peng|first7=Ying|last8=Li|first8=Yanli|last9=Jiang|first9=Xiaohua|last10=Huang|first10=Xuanlin|last11=Wang|first11=Jiaqian|last12=Wei|first12=Qing|last13=Qin|first13=Guijun|last14=Zhao|first14=Jiajun|last15=Jin|first15=Xiaolong|last16=Liu|first16=Li|last17=Li|first17=Yingrui|last18=Wang|first18=Weiqing|last19=Wang|first19=Jun|last20=Ning|first20=Guang|title=Whole exome sequencing of insulinoma reveals recurrent T372R mutations in YY1|journal=Nature Communications|volume=4|year=2013|issn=2041-1723|doi=10.1038/ncomms3810}}</ref>The understanding of role and functions of YY1 in β cells in near future might prove to be therapeutic potentials. | ||
*The progression to [[hypoglycemia]] is actually because of decreased glucose synthesis rather than increased use due to the direct effect of insulin on the liver.<ref name="RizzaHaymond1981">{{cite journal|last1=Rizza|first1=R. A.|last2=Haymond|first2=M. W.|last3=Verdonk|first3=C. A.|last4=Mandarino|first4=L. J.|last5=Miles|first5=J. M.|last6=Service|first6=F. J.|last7=Gerich|first7=J. E.|title=Pathogenesis of Hypoglycemia in Insulinoma Patients: Suppression of Hepatic Glucose Production by Insulin|journal=Diabetes|volume=30|issue=5|year=1981|pages=377–381|issn=0012-1797|doi=10.2337/diab.30.5.377}}</ref> | *The progression to [[hypoglycemia]] is actually because of decreased glucose synthesis rather than increased use due to the direct effect of [[insulin]] on the [[liver]].<ref name="RizzaHaymond1981">{{cite journal|last1=Rizza|first1=R. A.|last2=Haymond|first2=M. W.|last3=Verdonk|first3=C. A.|last4=Mandarino|first4=L. J.|last5=Miles|first5=J. M.|last6=Service|first6=F. J.|last7=Gerich|first7=J. E.|title=Pathogenesis of Hypoglycemia in Insulinoma Patients: Suppression of Hepatic Glucose Production by Insulin|journal=Diabetes|volume=30|issue=5|year=1981|pages=377–381|issn=0012-1797|doi=10.2337/diab.30.5.377}}</ref> | ||
*The neuroglycopenic symptoms appear eventually due to decreased blood glucose. Hypoglycemia stimulates catecholamine release which produces adrenergic symptoms.<ref name="pmid1305178">{{cite journal| author=Abe T| title=[Letter from Alabama--Medicaid and Medicare]. | journal=Kango | year= 1992 | volume= 44 | issue= 2 | pages= 135-40 | pmid=1305178 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=1305178 }} </ref> | *The neuroglycopenic symptoms appear eventually due to decreased [[blood glucose]]. [[Hypoglycemia]] stimulates [[catecholamine]] release which produces [[adrenergic]] symptoms.<ref name="pmid1305178">{{cite journal| author=Abe T| title=[Letter from Alabama--Medicaid and Medicare]. | journal=Kango | year= 1992 | volume= 44 | issue= 2 | pages= 135-40 | pmid=1305178 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=1305178 }} </ref> | ||
==Genetics== | ==Genetics== | ||
*Insulinoma is transmitted in an autosomal dominant pattern when it is associated with [[MEN 1 syndrome]]. | *Insulinoma is transmitted in an [[autosomal dominant]] pattern when it is associated with [[MEN 1 syndrome]]. | ||
*Genes involved in the pathogenesis of insulinoma include ''MEN1''gene. Loss of heterozygosity of ''MEN1'' gene takes place on chromosome | *Genes involved in the pathogenesis of insulinoma include ''MEN1''gene. [[Loss of heterozygosity]] of ''MEN1'' gene takes place on [[Chromosome 11 (human)|chromosome 11]]<nowiki/>q13<ref name="pmid20146582">{{cite journal |vauthors=Shin JJ, Gorden P, Libutti SK |title=Insulinoma: pathophysiology, localization and management |journal=Future Oncol |volume=6 |issue=2 |pages=229–37 |year=2010 |pmid=20146582 |pmc=3498768 |doi=10.2217/fon.09.165 |url=}}</ref> | ||
==Associated Conditions== | ==Associated Conditions== | ||
| Line 36: | Line 36: | ||
==Gross Pathology== | ==Gross Pathology== | ||
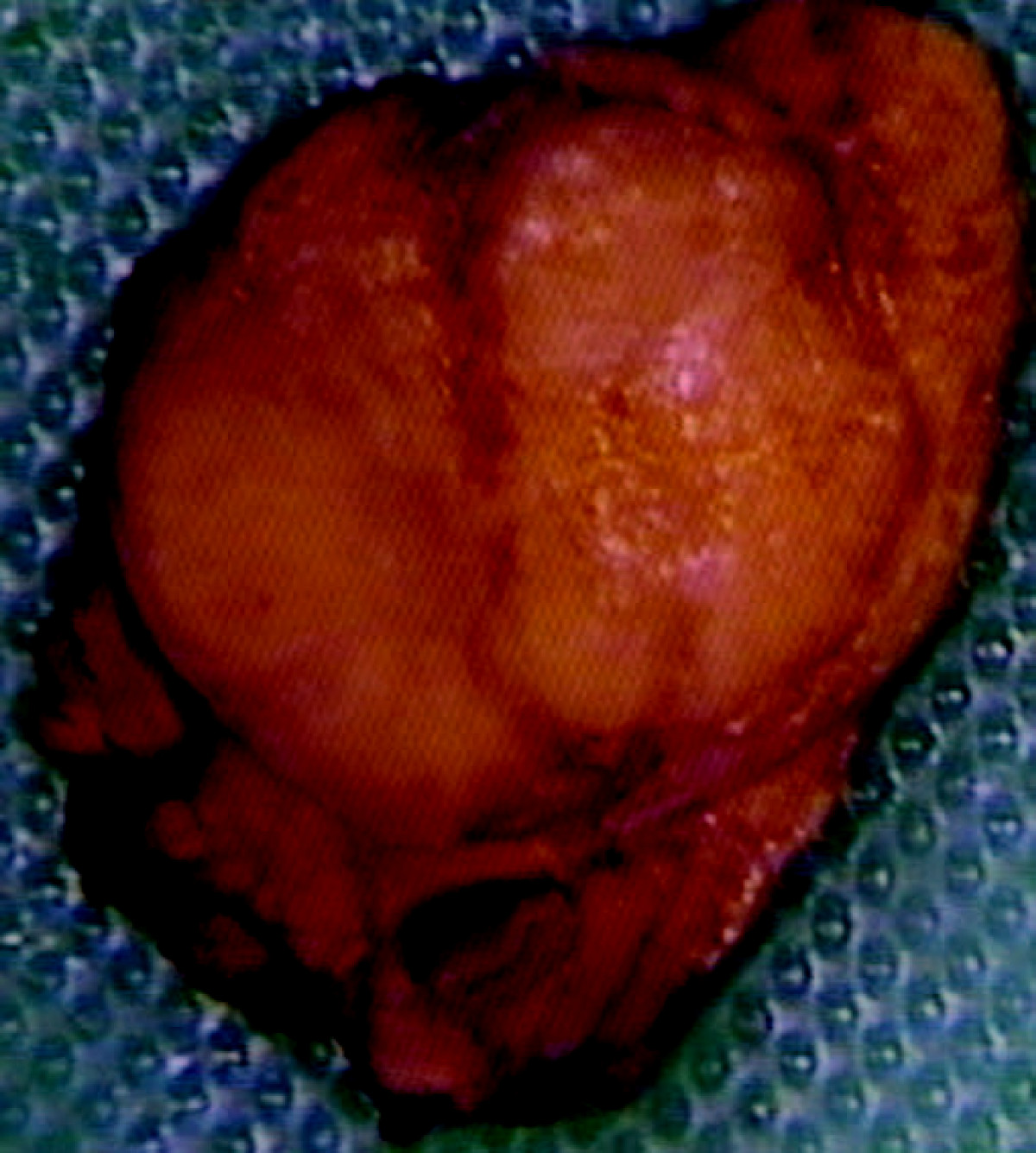
* On gross pathology insulinomas have a gray to red brown appearance, encapsulated <ref>{{cite book | last = Lloyd | first = Ricardo | title = Endocrine pathology : differential diagnosis and molecular advances | publisher = Springer | location = New York London | year = 2010 | isbn = 978-1441910684 }}</ref>and are usually small and solitary tumors. Although there is a case report of a large(9cm), pedunculated and weighing more than 100g.<ref name="pmid15522939">{{cite journal| author=Mittendorf EA, Liu YC, McHenry CR| title=Giant insulinoma: case report and review of the literature. | journal=J Clin Endocrinol Metab | year= 2005 | volume= 90 | issue= 1 | pages= 575-80 | pmid=15522939 | doi=10.1210/jc.2004-0825 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15522939 }} </ref> | * On gross pathology insulinomas have a gray to red brown appearance, encapsulated <ref>{{cite book | last = Lloyd | first = Ricardo | title = Endocrine pathology : differential diagnosis and molecular advances | publisher = Springer | location = New York London | year = 2010 | isbn = 978-1441910684 }}</ref>and are usually small and solitary tumors. Although there is a case report of a large(9cm), [[pedunculated]] and weighing more than 100g.<ref name="pmid15522939">{{cite journal| author=Mittendorf EA, Liu YC, McHenry CR| title=Giant insulinoma: case report and review of the literature. | journal=J Clin Endocrinol Metab | year= 2005 | volume= 90 | issue= 1 | pages= 575-80 | pmid=15522939 | doi=10.1210/jc.2004-0825 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15522939 }} </ref> | ||
[[Image:Insulinoma.jpg|thumb|center|250px|Insulinoma- Red brown appearance. By Edward Alabraba et al. - Pancreatic insulinoma co-existing with gastric GIST in the absence of neurofibromatosis-1. World Journal of Surgical Oncology 2009, 7:18doi:10.1186/1477-7819-7-18, CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=6686376]] | [[Image:Insulinoma.jpg|thumb|center|250px|Insulinoma- Red brown appearance. By Edward Alabraba et al. - Pancreatic insulinoma co-existing with gastric GIST in the absence of neurofibromatosis-1. World Journal of Surgical Oncology 2009, 7:18doi:10.1186/1477-7819-7-18, CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=6686376]] | ||
Revision as of 14:06, 21 August 2017
|
Insulinoma Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Insulinoma pathophysiology On the Web |
|
American Roentgen Ray Society Images of Insulinoma pathophysiology |
|
Risk calculators and risk factors for Insulinoma pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]Associate Editor(s)-in-Chief: Amandeep Singh M.D.[2]
Overview
- It is thought that [disease name] is the result of / is mediated by / is produced by / is caused by either [hypothesis 1], [hypothesis 2], or [hypothesis 3].
- Insulinoma arises from β islet cells, which are endocrine cells that are normally involved in the production of insulin.
- The progression to [disease name] usually involves the [molecular pathway].
- The pathophysiology of [disease/malignancy] depends on the histological subtype.
Pathophysiology
Pathogenesis
- Insulinoma is a rare benign pancreatic neuroendocrine tumor that arises from β islet cells, which are cells that are normally involved in the production of insulin. Few insulinomas can also produce other hormones such as Serotonin, gastrin, ACTH, glucagon, and somatostatin [1]
- They are usually small(90%), sporadic(90%), solitary(90%) and benign(90%) tumors.
- It usually occurs sporadically but 10% are found to be associated with MEN 1 syndrome.[2]Those associated with the MEN1 syndrome are usually malignant and higher recurrence rate(21% at 10 and 20 years) than in those without MEN 1 (5% at 10 and 7% at 20 years). [3].
- It is thought that insulinoma is mediated by mTOR/P70S6K signaling pathway. Inhibitors of mTOR(rapamycin) or dual PI3K/mTOR(NVP-BEZ2235)thus have become new drugs for treating insulinoma. An oral mTOR inhibitor, Everolimus, make better glycemic control in people having insulinoma.[4] The pathway will give more possibilities for medical treatment.[5]
- Mitochondria play a key role in glucose and insulin coupling to assure insulin secretion after glucose stimulation in pancreatic β cells. Coupling is impaired due to abnormal mitochondrial function in β cells causes the death of the cell.[6]YY1 regulates this mitochondrial function.[7]T372R mutation increase the transcription of YY1.[8]The understanding of role and functions of YY1 in β cells in near future might prove to be therapeutic potentials.
- The progression to hypoglycemia is actually because of decreased glucose synthesis rather than increased use due to the direct effect of insulin on the liver.[9]
- The neuroglycopenic symptoms appear eventually due to decreased blood glucose. Hypoglycemia stimulates catecholamine release which produces adrenergic symptoms.[10]
Genetics
- Insulinoma is transmitted in an autosomal dominant pattern when it is associated with MEN 1 syndrome.
- Genes involved in the pathogenesis of insulinoma include MEN1gene. Loss of heterozygosity of MEN1 gene takes place on chromosome 11q13[11]
Associated Conditions
- Other pancreatic neuroendocrine tumors like gastrinoma, glucagonoma, VIPoma, somatostatinoma
- MEN 1[3]
- von Hippel-Lindau
- Neurofibromatosis type 1
Gross Pathology
- On gross pathology insulinomas have a gray to red brown appearance, encapsulated [12]and are usually small and solitary tumors. Although there is a case report of a large(9cm), pedunculated and weighing more than 100g.[13]
- Almost all insulinomas are present throughout the pancreas and extrapancreatic ones causing hypoglycemia are rare(<2%)[14]
- Various other findings are noted on gross pathology like[15]
- size of the tumor
- metastasis to lymph nodes
- extrapancreatic involvement
- distant metastasis
Microscopic Pathology
- On microscopic histopathological analysis,patterns like trabecular, gyriform, lobular and solid structures particularly with amyloid in fibrovascular stroma are characteristic findings of insulinoma.[16]
- We also evaluate for the mitotic index(mitosis per 10 high power field) and immunohistochemistry staining by Chromogranin A, synaptophysin, and Ki-67 index.[15]
-
Histopathology of pancreatic endocrine tumor (insulinoma). H&E stain[17]
-
Histopathology of pancreatic endocrine tumor (insulinoma)[17]
-
Histopathology of pancreatic endocrine tumor (insulinoma). Chromogranin A immunostain[17]
-
Histopathology of pancreatic endocrine tumor (insulinoma). Insulin immunostain[17]
References
- ↑ AlJadir, Saadi (2015). "Insulinoma: Literature's Review (Part 1)". Endocrinology&Metabolism International Journal. 2 (3). doi:10.15406/emij.2015.02.00025. ISSN 2473-0815.
- ↑ Callender GG, Rich TA, Perrier ND (2008). "Multiple endocrine neoplasia syndromes". Surg Clin North Am. 88 (4): 863–95, viii. doi:10.1016/j.suc.2008.05.001. PMID 18672144.
- ↑ 3.0 3.1 Service FJ, McMahon MM, O'Brien PC, Ballard DJ (1991). "Functioning insulinoma--incidence, recurrence, and long-term survival of patients: a 60-year study". Mayo Clin Proc. 66 (7): 711–9. PMID 1677058.
- ↑ Kulke MH, Bergsland EK, Yao JC (2009). "Glycemic control in patients with insulinoma treated with everolimus". N Engl J Med. 360 (2): 195–7. doi:10.1056/NEJMc0806740. PMID 19129539.
- ↑ Zhan HX, Cong L, Zhao YP, Zhang TP, Chen G, Zhou L; et al. (2012). "Activated mTOR/P70S6K signaling pathway is involved in insulinoma tumorigenesis". J Surg Oncol. 106 (8): 972–80. doi:10.1002/jso.23176. PMID 22711648.
- ↑ Supale S, Li N, Brun T, Maechler P (2012). "Mitochondrial dysfunction in pancreatic β cells". Trends Endocrinol Metab. 23 (9): 477–87. doi:10.1016/j.tem.2012.06.002. PMID 22766318.
- ↑ Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P (2007). "mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex". Nature. 450 (7170): 736–40. doi:10.1038/nature06322. PMID 18046414.
- ↑ Cao, Yanan; Gao, Zhibo; Li, Lin; Jiang, Xiuli; Shan, Aijing; Cai, Jie; Peng, Ying; Li, Yanli; Jiang, Xiaohua; Huang, Xuanlin; Wang, Jiaqian; Wei, Qing; Qin, Guijun; Zhao, Jiajun; Jin, Xiaolong; Liu, Li; Li, Yingrui; Wang, Weiqing; Wang, Jun; Ning, Guang (2013). "Whole exome sequencing of insulinoma reveals recurrent T372R mutations in YY1". Nature Communications. 4. doi:10.1038/ncomms3810. ISSN 2041-1723.
- ↑ Rizza, R. A.; Haymond, M. W.; Verdonk, C. A.; Mandarino, L. J.; Miles, J. M.; Service, F. J.; Gerich, J. E. (1981). "Pathogenesis of Hypoglycemia in Insulinoma Patients: Suppression of Hepatic Glucose Production by Insulin". Diabetes. 30 (5): 377–381. doi:10.2337/diab.30.5.377. ISSN 0012-1797.
- ↑ Abe T (1992). "[Letter from Alabama--Medicaid and Medicare]". Kango. 44 (2): 135–40. PMID 1305178.
- ↑ Shin JJ, Gorden P, Libutti SK (2010). "Insulinoma: pathophysiology, localization and management". Future Oncol. 6 (2): 229–37. doi:10.2217/fon.09.165. PMC 3498768. PMID 20146582.
- ↑ Lloyd, Ricardo (2010). Endocrine pathology : differential diagnosis and molecular advances. New York London: Springer. ISBN 978-1441910684.
- ↑ Mittendorf EA, Liu YC, McHenry CR (2005). "Giant insulinoma: case report and review of the literature". J Clin Endocrinol Metab. 90 (1): 575–80. doi:10.1210/jc.2004-0825. PMID 15522939.
- ↑ Okabayashi T, Shima Y, Sumiyoshi T, Kozuki A, Ito S, Ogawa Y, Kobayashi M, Hanazaki K (2013). "Diagnosis and management of insulinoma". World J. Gastroenterol. 19 (6): 829–37. doi:10.3748/wjg.v19.i6.829. PMC 3574879. PMID 23430217.
- ↑ 15.0 15.1 de Herder, Wouter W.; Niederle, Bruno; Scoazec, Jean-Yves; Pauwels, Stanislas; Klöppel, Günter; Falconi, Massimo; Kwekkeboom, Dik J.; Öberg, Kjel; Eriksson, Barbro; Wiedenmann, Bertram; Rindi, Guido; O’Toole, Dermot; Ferone, Diego (2007). "Well-Differentiated Pancreatic Tumor/Carcinoma: Insulinoma". Neuroendocrinology. 84 (3): 183–188. doi:10.1159/000098010. ISSN 0028-3835.
- ↑ Lloyd, Ricardo (2010). Endocrine pathology : differential diagnosis and molecular advances. New York London: Springer. ISBN 978-1441910684.
- ↑ 17.0 17.1 17.2 17.3 Neuroendocrine tumour of the pancreas. Libre Pathology. http://librepathology.org/wiki/index.php/Neuroendocrine_tumour_of_the_pancreas
![Histopathology of pancreatic endocrine tumor (insulinoma). H&E stain[17]](/images/2/2d/Pancreatic_insulinoma_Histology_1.JPG)
![Histopathology of pancreatic endocrine tumor (insulinoma)[17]](/images/2/2f/Pancreatic_insulinoma_histology_2.JPG)
![Histopathology of pancreatic endocrine tumor (insulinoma). Chromogranin A immunostain[17]](/images/a/a3/Pancreatic_insulinoma_histopathology_3.JPG)
![Histopathology of pancreatic endocrine tumor (insulinoma). Insulin immunostain[17]](/images/d/d5/Pancreatic_insulinoma_histology_4.JPG)