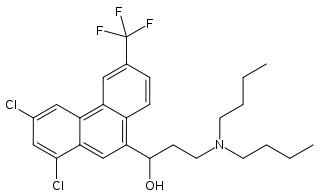
Halofantrine
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a603030 |
| Routes of administration | Oral |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | 60 to 70% |
| Metabolism | Hepatic (CYP3A4-mediated) |
| Elimination half-life | 6 to 10 days |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C26H30Cl2F3NO |
| Molar mass | 500.423 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
|
WikiDoc Resources for Halofantrine |
|
Articles |
|---|
|
Most recent articles on Halofantrine Most cited articles on Halofantrine |
|
Media |
|
Powerpoint slides on Halofantrine |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Halofantrine at Clinical Trials.gov Clinical Trials on Halofantrine at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Halofantrine
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Halofantrine Discussion groups on Halofantrine Patient Handouts on Halofantrine Directions to Hospitals Treating Halofantrine Risk calculators and risk factors for Halofantrine
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Halofantrine |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Halofantrine is a drug used to treat malaria. Halofantrine's structure contains a substituted phenanthrene, and is related to the antimalarial drugs quinine and lumefantrine. Marketed as Halfan, halofantrine is never used to prevent malaria and its mode of action is unknown, although a crystallographic study showed that it binds to hematin in vitro, suggesting a possible mechanism of action.[1] Halofantrine has also been shown to bind to plasmpesin, a haemoglobin degrading enzyme unique to the malarial parasites.[2]
Halofantrine was developed at SRI International for the Walter Reed Army Institute of Research from 1965 to 1975 by a team led by medicinal chemist William Colwell.[3]
Adverse reactions
Halofantrine can cause abdominal pain, diarrhea, vomiting, rash, headache, itching and elevated liver enzymes.
It can be associated with cardiotoxicity.[4] The most dangerous side effect is cardiac arrhythmias: halofantrine causes significant QT prolongation,[5] and this effect is seen even at standard doses. The drug should therefore not be given to patients with cardiac conduction defects and should not be combined with mefloquine. A survey from 2009 suggests that the drug is safe when correctly administered [6]it
Other adverse reactions
Consumption of grapefruit combined with certain medications can cause serious side effects, even death. Halofantrine combined with this fruit or grapefruit juice is dangerous. The mechanism of action is inhibition of CYP3A4, which is necessary to metabolize the drug and eliminate it from the body. Without CYP3A4, levels of the drug will become toxic in the body.
Pharmacology
The mechanism of action of halofantrine is unknown. The absorption of halofantrine is erratic, but is increased when taken with fatty food. Because of fears of toxicity due to increased halofantrine blood levels, halofantrine should be taken on an empty stomach.
Plasma levels peak at 16 hours and the half-life of the drug is about 4 days.
Uses
Halofantrine is only used to treat malaria. It is not used to prevent malaria (prophylaxis) because of the risk of toxicity and unreliable absorption.
Dosing
Adult dose: Three doses of 500 mg six hours apart. Halofantrine should be taken on an empty stomach.
Manufacturing information and availability
Halfan (GlaxoSmithKline) is available as 250 mg tablets. A full treatment cost (6 tablets) costs US$1.40 in the developing world. Halofantrine is not available in the UK or U.S.
Synthesis

References
- ↑ de Villiers KA, Marques HM, Egan TJ (2008). "The crystal structure of halofantrine-ferriprotoporphyrin IX and the mechanism of action of arylmethanol antimalarials". J. Inorg. Chem. 102 (8): 1660–7. doi:10.1016/j.jinorgbio.2008.04.001. PMID 18508124.
- ↑ Friedman R, Caflisch A (2009). "Discovery of plasmepsin inhibitors by fragment-based docking and consensus scoring". ChemMedChem. 4 (8): 1317–26. doi:10.1002/cmdc.200900078. PMID 19472268.
- ↑ Nielson, Donald (2006). A Heritage of Innovation: SRI's First Half Century. Menlo Park, California: SRI International. pp. 10-3–10-5. ISBN 978-0974520810.
- ↑ Wesche DL, Schuster BG, Wang WX, Woosley RL (May 2000). "Mechanism of cardiotoxicity of halofantrine". Clin. Pharmacol. Ther. 67 (5): 521–9. doi:10.1067/mcp.2000.106127. PMID 10824631.
- ↑ Sánchez-Chapula JA, Navarro-Polanco RA, Sanguinetti MC (2004). "Block of wild-type and inactivation-deficient human ether-a-go-go-related gene K+ channels by halofantrine". Naunyn Schmiedebergs Arch. Pharmacol. 370 (6): 484–91. doi:10.1007/s00210-004-0995-5. PMID 15558243.
- ↑ Bouchaud O, Imbert P, Touze JE, Dodoo AN, Danis M, Legros F (2009). "Fatal cardiotoxicity related to halofantrine: a review based on a worldwide safety data base". Malaria J. 8: 289. doi:10.1186/1475-2875-8-289. PMC 2801676. PMID 20003315.
- Pages with script errors
- CS1 maint: Multiple names: authors list
- Template:drugs.com link with non-standard subpage
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without KEGG source
- Drugs with no legal status
- Antimalarial agents
- Organochlorides
- Organofluorides
- Alcohols
- Drug