HPV Vaccine: Difference between revisions
No edit summary |
No edit summary |
||
| Line 170: | Line 170: | ||
=====Use with Systemic Immunosuppressive Medications===== | =====Use with Systemic Immunosuppressive Medications===== | ||
Immunosuppressive therapies, including irradiation, antimetabolites, alkylating agents, cytotoxic drugs, and corticosteroids (used in greater than physiologic doses), may reduce the immune responses to vaccines | Immunosuppressive therapies, including irradiation, antimetabolites, alkylating agents, cytotoxic drugs, and corticosteroids (used in greater than physiologic doses), may reduce the immune responses to vaccines | ||
|useInPregnancyFDA=( | |FDAPregCat=B | ||
|useInPregnancyFDA=Reproduction studies have been performed in female rats at doses equivalent to the recommended human dose and have revealed no evidence of impaired female fertility or harm to the fetus due to GARDASIL. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human responses, GARDASIL should be used during pregnancy only if clearly needed. | |||
An evaluation of the effect of GARDASIL on embryo-fetal, pre- and postweaning development was conducted using rats. One group of rats was administered GARDASIL twice prior to gestation, during the period of organogenesis (gestation Day 6) and on lactation Day 7. A second group of pregnant rats was administered GARDASIL during the period of organogenesis (gestation Day 6) and on lactation Day 7 only. GARDASIL was administered at 0.5 mL/rat/occasion (120 mcg total protein which is equivalent to the recommended human dose) by intramuscular injection. No adverse effects on mating, fertility, pregnancy, parturition, lactation, embryo-fetal or pre- and postweaning development were observed. There were no vaccine-related fetal malformations or other evidence of teratogenesis noted in this study. In addition, there were no treatment-related effects on developmental signs, behavior, reproductive performance, or fertility of the offspring. | |||
=====Clinical Studies in Humans===== | |||
In clinical studies, women underwent urine pregnancy testing prior to administration of each dose of GARDASIL. Women who were found to be pregnant before completion of a 3-dose regimen of GARDASIL were instructed to defer completion of their vaccination regimen until resolution of the pregnancy. | |||
GARDASIL is not indicated for women 27 years of age or older. However, safety data in women 16 through 45 years of age was collected, and 3819 women (GARDASIL N = 1894 vs. AAHS control or saline placebo N = 1925) reported at least 1 pregnancy each. | |||
| | |||
The overall proportions of pregnancies that resulted in an adverse outcome, defined as the combined numbers of spontaneous abortion, late fetal death, and congenital anomaly cases out of the total number of pregnancy outcomes for which an outcome was known (and excluding elective terminations), were 22.6% (446/1973) in women who received GARDASIL and 23.1% (460/1994) in women who received AAHS control or saline placebo. | |||
| | Overall, 55 and 65 women in the group that received GARDASIL or AAHS control or saline placebo, respectively (2.9% and 3.4% of all women who reported a pregnancy in the respective vaccination groups), experienced a serious adverse reaction during pregnancy. The most common events reported were conditions that can result in Caesarean section (e.g., failure of labor, malpresentation, cephalopelvic disproportion), premature onset of labor (e.g., threatened abortions, premature rupture of membranes), and pregnancy-related medical problems (e.g., pre-eclampsia, hyperemesis). The proportions of pregnant women who experienced such events were comparable between the groups receiving GARDASIL and AAHS control or saline placebo. | ||
| | |||
| | There were 45 cases of congenital anomaly in pregnancies that occurred in women who received GARDASIL and 34 cases of congenital anomaly in pregnancies that occurred in women who received AAHS control or saline placebo. | ||
Further sub-analyses were conducted to evaluate pregnancies with estimated onset within 30 days or more than 30 days from administration of a dose of GARDASIL or AAHS control or saline placebo. For pregnancies with estimated onset within 30 days of vaccination, 5 cases of congenital anomaly were observed in the group that received GARDASIL compared to 1 case of congenital anomaly in the group that received AAHS control or saline placebo. The congenital anomalies seen in pregnancies with estimated onset within 30 days of vaccination included pyloric stenosis, congenital megacolon, congenital hydronephrosis, hip dysplasia, and club foot. Conversely, in pregnancies with onset more than 30 days following vaccination, 40 cases of congenital anomaly were observed in the group that received GARDASIL compared with 33 cases of congenital anomaly in the group that received AAHS control or saline placebo. | |||
|useInNursing======Women 16 Through 45 Years of Age===== | |||
It is not known whether GARDASIL is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when GARDASIL is administered to a nursing woman. | |||
GARDASIL or AAHS control were given to a total of 1133 women (vaccine N = 582, AAHS control N = 551) during the relevant Phase III clinical studies. | |||
Overall, 27 and 13 infants of women who received GARDASIL or AAHS control, respectively (representing 4.6% and 2.4% of the total number of women who were breast-feeding during the period in which they received GARDASIL or AAHS control, respectively), experienced a serious adverse reaction. | |||
In a post-hoc analysis of clinical studies, a higher number of breast-feeding infants (n = 7) whose mothers received GARDASIL had acute respiratory illnesses within 30 days post vaccination of the mother as compared to infants (n = 2) whose mothers received AAHS control. | |||
|useInPed=Safety and effectiveness have not been established in pediatric patients below 9 years of age. | |||
|useInGeri=The safety and effectiveness of GARDASIL have not been evaluated in a geriatric population, defined as individuals aged 65 years and over. | |||
|useInImmunocomp=The immunologic response to GARDASIL may be diminished in immunocompromised individuals | |||
|administration=(Oral/Intravenous/etc) | |administration=(Oral/Intravenous/etc) | ||
|monitoring======Condition 1===== | |monitoring======Condition 1===== | ||
Revision as of 15:24, 25 August 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alonso Alvarado, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
HPV Vaccine is a Adrenergic receptor agonist that is FDA approved for the prophylaxis of HPV types 6, 11, 16 and 18 infections. Common adverse reactions include erythema at injection site, injection site pain, injection site pruritus, swelling at injection site, nausea, dizziness, headache, fever.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Dosage
GARDASIL should be administered intramuscularly as a 0.5-mL dose at the following schedule: 0, 2 months, 6 months. [See Clinical Studies (14.8).]
Method of Administration
For intramuscular use only. Shake well before use. Thorough agitation immediately before administration is necessary to maintain suspension of the vaccine. GARDASIL should not be diluted or mixed with other vaccines. After thorough agitation, GARDASIL is a white, cloudy liquid. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. Do not use the product if particulates are present or if it appears discolored. GARDASIL should be administered intramuscularly in the deltoid region of the upper arm or in the higher anterolateral area of the thigh. Syncope has been reported following vaccination with GARDASIL and may result in falling with injury; observation for 15 minutes after administration is recommended.
Single-Dose Vial Use
Withdraw the 0.5-mL dose of vaccine from the single-dose vial using a sterile needle and syringe and use promptly.
Prefilled Syringe Use
This package does not contain a needle. Shake well before use. Attach the needle by twisting in a clockwise direction until the needle fits securely on the syringe. Administer the entire dose as per standard protocol.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of HPV Vaccine in adult patients.
Non–Guideline-Supported Use
Prophylaxis of Adenocarcinoma in situ of cervix, due to Nonvaccine HPV types
- Dosing Information
Prophylaxis Cervical intraepithelial neoplasia, Grade 1, 2, and 3 due to Nonvaccine Human Papillomavirus Types
- Dosing Information
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding HPV Vaccine FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of HPV Vaccine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of HPV Vaccine in pediatric patients.
Contraindications
Hypersensitivity, including severe allergic reactions to yeast (a vaccine component), or after a previous dose of GARDASIL.
Warnings
Syncope
Because vaccinees may develop syncope, sometimes resulting in falling with injury, observation for 15 minutes after administration is recommended. Syncope, sometimes associated with tonic-clonic movements and other seizure-like activity, has been reported following vaccination with GARDASIL. When syncope is associated with tonic-clonic movements, the activity is usually transient and typically responds to restoring cerebral perfusion by maintaining a supine or Trendelenburg position.
Managing Allergic Reactions
Appropriate medical treatment and supervision must be readily available in case of anaphylactic reactions following the administration of GARDASIL.
Adverse Reactions
Clinical Trials Experience
Overall Summary of Adverse Reactions
Headache, fever, nausea, and dizziness; and local injection site reactions (pain, swelling, erythema, pruritus, and bruising) occurred after administration with GARDASIL.
Syncope, sometimes associated with tonic-clonic movements and other seizure-like activity, has been reported following vaccination with GARDASIL and may result in falling with injury; observation for 15 minutes after administration is recommended.
Anaphylaxis has been reported following vaccination with GARDASIL.
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect the rates observed in practice.
Studies in Girls and Women (9 Through 45 Years of Age) and Boys and Men (9 Through 26 Years of Age)
In 7 clinical trials (5 Amorphous Aluminum Hydroxyphosphate Sulfate [AAHS]-controlled, 1 saline placebo-controlled, and 1 uncontrolled), 18,083 individuals were administered GARDASIL or AAHS control or saline placebo on the day of enrollment, and approximately 2 and 6 months thereafter, and safety was evaluated using vaccination report cards (VRC)-aided surveillance for 14 days after each injection of GARDASIL or AAHS control or saline placebo in these individuals. The individuals who were monitored using VRC-aided surveillance included 10,088 individuals 9 through 45 years of age at enrollment who received GARDASIL and 7,995 individuals who received AAHS control or saline placebo. Few individuals (0.2%) discontinued due to adverse reactions. The race distribution of the 9- through 26-year-old girls and women in the safety population was as follows: 62.3% White; 17.6% Hispanic (Black and White); 6.8% Asian; 6.7% Other; 6.4% Black; and 0.3% American Indian. The race distribution of the 24- through 45-year-old women in the safety population of Study 6 was as follows: 20.6% White; 43.2% Hispanic (Black and White); 0.2% Other; 4.8% Black; 31.2% Asian; and 0.1% American Indian. The race distribution of the 9- through 26-year-old boys and men in the safety population was as follows: 42.0% White; 19.7% Hispanic (Black and White); 11.0% Asian; 11.2% Other; 15.9% Black; and 0.1% American Indian.
Common Injection-Site Adverse Reactions in Girls and Women 9 Through 26 Years of Age
The injection site adverse reactions that were observed among recipients of GARDASIL at a frequency of at least 1.0% and also at a greater frequency than that observed among AAHS control or saline placebo recipients are shown in Table 1.
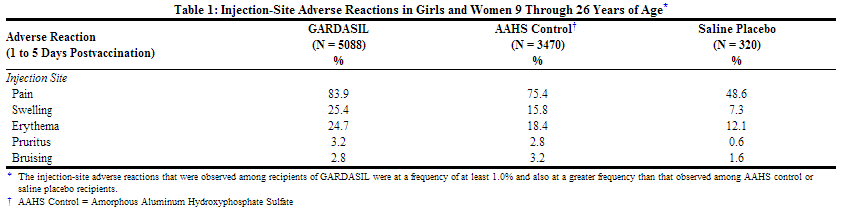
Common Injection-Site Adverse Reactions in Boys and Men 9 Through 26 Years of Age
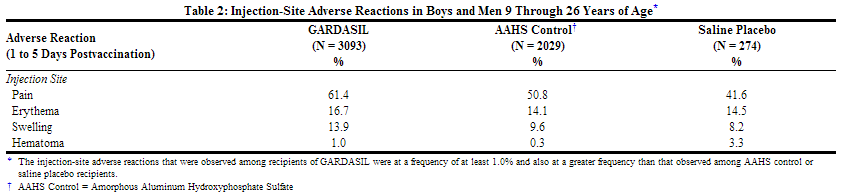
The injection site adverse reactions that were observed among recipients of GARDASIL at a frequency of at least 1.0% and also at a greater frequency than that observed among AAHS control or saline placebo recipients are shown in Table 2.
Evaluation of Injection-Site Adverse Reactions by Dose in Girls and Women 9 Through 26 Years of Age
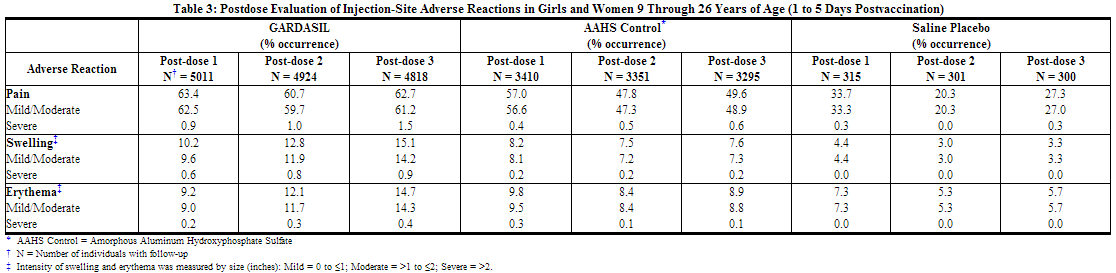
An analysis of injection-site adverse reactions in girls and women by dose is shown in Table 3. Of those girls and women who reported an injection-site reaction, 94.3% judged their injection-site adverse reaction to be mild or moderate in intensity.
Evaluation of Injection-Site Adverse Reactions by Dose in Boys and Men 9 Through 26 Years of Age
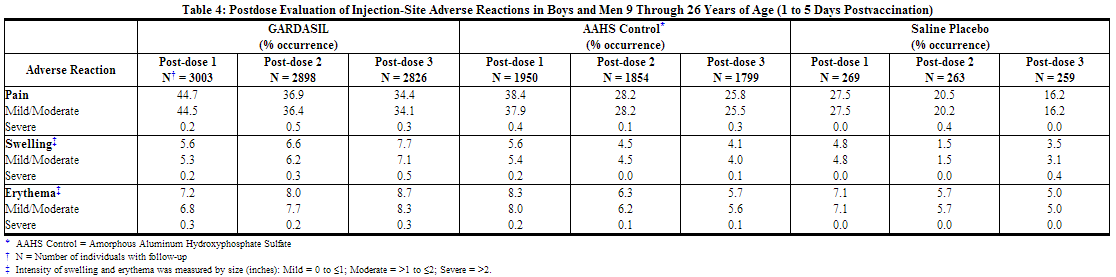
An analysis of injection-site adverse reactions in boys and men by dose is shown in Table 4. Of those boys and men who reported an injection-site reaction, 96.4% judged their injection-site adverse reaction to be mild or moderate in intensity.
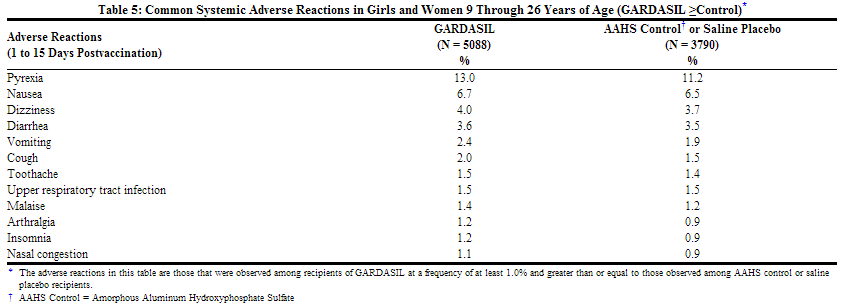
Common Systemic Adverse Reactions in Girls and Women 9 Through 26 Years of Age
Headache was the most commonly reported systemic adverse reaction in both treatment groups (GARDASIL = 28.2% and AAHS control or saline placebo = 28.4%). Fever was the next most commonly reported systemic adverse reaction in both treatment groups (GARDASIL = 13.0% and AAHS control or saline placebo = 11.2%).
Adverse reactions that were observed among recipients of GARDASIL, at a frequency of greater than or equal to 1.0% where the incidence in the GARDASIL group was greater than or equal to the incidence in the AAHS control or saline placebo group, are shown in Table 5.
Common Systemic Adverse Reactions in Boys and Men 9 Through 26 Years of Age
Headache was the most commonly reported systemic adverse reaction in both treatment groups (GARDASIL = 12.3% and AAHS control or saline placebo = 11.2%). Fever was the next most commonly reported systemic adverse reaction in both treatment groups (GARDASIL = 8.3% and AAHS control or saline placebo = 6.5%).
Adverse reactions that were observed among recipients of GARDASIL, at a frequency of greater than or equal to 1.0% where the incidence in the group that received GARDASIL was greater than or equal to the incidence in the AAHS control or saline placebo group, are shown in Table 6.
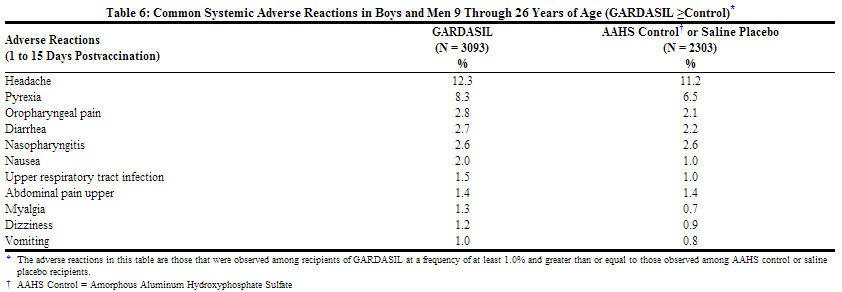
Evaluation of Fever by Dose in Girls and Women 9 Through 26 Years of Age
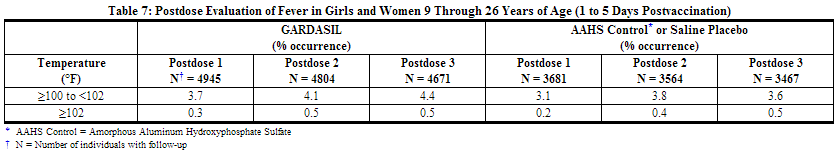
An analysis of fever in girls and women by dose is shown in Table 7.
Evaluation of Fever by Dose in Boys and Men 9 Through 26 Years of Age
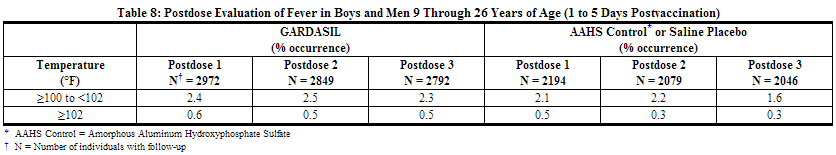
An analysis of fever in boys and men by dose is shown in Table 8.
Serious Adverse Reactions in the Entire Study Population
Across the clinical studies, 258 individuals (GARDASIL N = 128 or 0.8%; placebo N = 130 or 1.0%) out of 29,323 (GARDASIL N = 15,706; AAHS control N = 13,023; or saline placebo N = 594) individuals (9- through 45-year-old girls and women; and 9- through 26-year-old boys and men) reported a serious systemic adverse reaction. Of the entire study population (29,323 individuals), 0.04% of the reported serious systemic adverse reactions were judged to be vaccine related by the study investigator. The most frequently (frequency of 4 cases or greater with either GARDASIL, AAHS control, saline placebo, or the total of all three) reported serious systemic adverse reactions, regardless of causality, were: Headache [0.02% GARDASIL (3 cases) vs. 0.02% AAHS control (2 cases)], Gastroenteritis [0.02% GARDASIL (3 cases) vs. 0.02% AAHS control (2 cases)], Appendicitis [0.03% GARDASIL (5 cases) vs. 0.01% AAHS control (1 case)], Pelvic inflammatory disease [0.02% GARDASIL (3 cases) vs. 0.03% AAHS control (4 cases)], Urinary tract infection [0.01% GARDASIL (2 cases) vs. 0.02% AAHS control (2 cases)], Pneumonia [0.01% GARDASIL (2 cases) vs. 0.02% AAHS control (2 cases)], Pyelonephritis [0.01% GARDASIL (2 cases) vs. 0.02% AAHS control (3 cases)], Pulmonary embolism [0.01% GARDASIL (2 cases) vs. 0.02% AAHS control (2 cases)]. One case (0.006% GARDASIL; 0.0% AAHS control or saline placebo) of bronchospasm; and 2 cases (0.01% GARDASIL; 0.0% AAHS control or saline placebo) of asthma were reported as serious systemic adverse reactions that occurred following any vaccination visit. In addition, there was 1 individual in the clinical trials, in the group that received GARDASIL, who reported two injection-site serious adverse reactions (injection-site pain and injection-site joint movement impairment).
Deaths in the Entire Study Population
Across the clinical studies, 40 deaths (GARDASIL N = 21 or 0.1%; placebo N = 19 or 0.1%) were reported in 29,323 (GARDASIL N = 15,706; AAHS control N = 13,023, saline placebo N = 594) individuals (9- through 45-year-old girls and women; and 9- through 26-year-old boys and men). The events reported were consistent with events expected in healthy adolescent and adult populations. The most common cause of death was motor vehicle accident (5 individuals who received GARDASIL and 4 individuals who received AAHS control), followed by drug overdose/suicide (2 individuals who received GARDASIL and 6 individuals who received AAHS control), gunshot wound (1 individual who received GARDASIL and 3 individuals who received AAHS control), and pulmonary embolus/deep vein thrombosis (1 individual who received GARDASIL and 1 individual who received AAHS control). In addition, there were 2 cases of sepsis, 1 case of pancreatic cancer, 1 case of arrhythmia, 1 case of pulmonary tuberculosis, 1 case of hyperthyroidism, 1 case of post-operative pulmonary embolism and acute renal failure, 1 case of traumatic brain injury/cardiac arrest, 1 case of systemic lupus erythematosus, 1 case of cerebrovascular accident, 1 case of breast cancer, and 1 case of nasopharyngeal cancer in the group that received GARDASIL; 1 case of asphyxia, 1 case of acute lymphocytic leukemia, 1 case of chemical poisoning, and 1 case of myocardial ischemia in the AAHS control group; and 1 case of medulloblastoma in the saline placebo group.
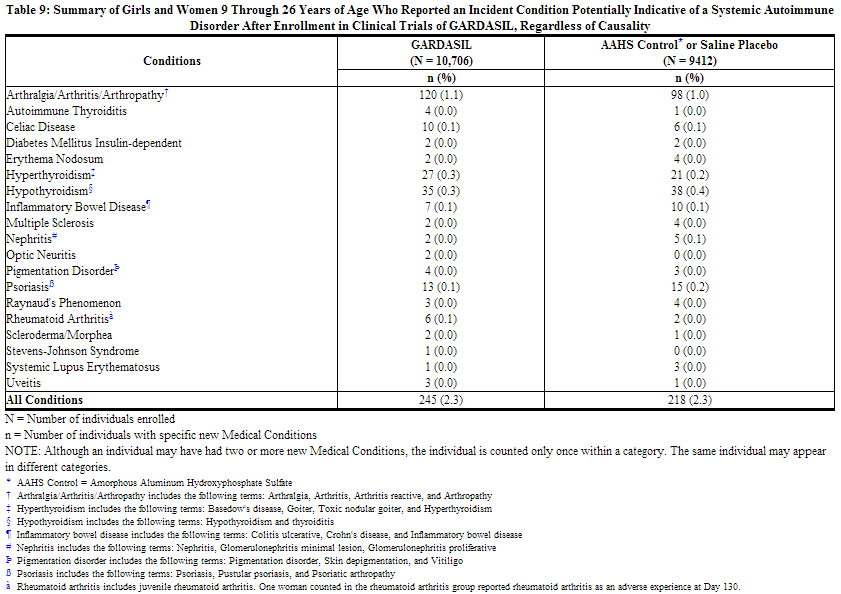
Systemic Autoimmune Disorders in Girls and Women 9 Through 26 Years of Age
In the clinical studies, 9- through 26-year-old girls and women were evaluated for new medical conditions that occurred over the course of follow-up. New medical conditions potentially indicative of a systemic autoimmune disorder seen in the group that received GARDASIL or AAHS control or saline placebo are shown in Table 9. This population includes all girls and women who received at least one dose of GARDASIL or AAHS control or saline placebo, and had safety data available.
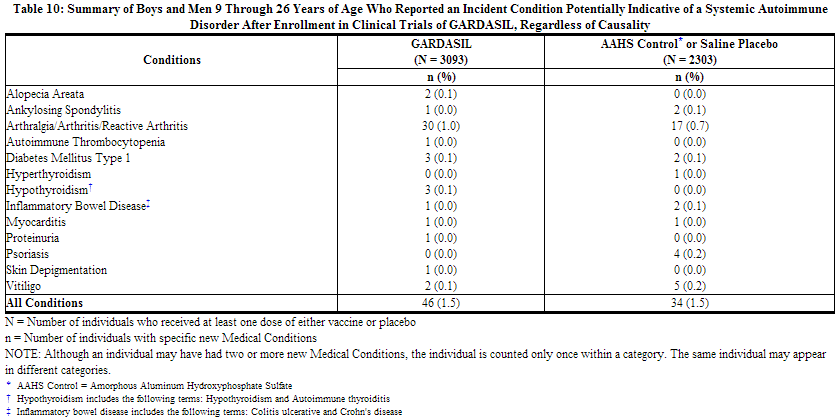
Systemic Autoimmune Disorders in Boys and Men 9 Through 26 Years of Age
In the clinical studies, 9- through 26-year-old boys and men were evaluated for new medical conditions that occurred over the course of follow-up. New medical conditions potentially indicative of a systemic autoimmune disorder seen in the group that received GARDASIL or AAHS control or saline placebo are shown in Table 10. This population includes all boys and men who received at least one dose of GARDASIL or AAHS control or saline placebo, and had safety data available.
Safety in Concomitant Use with RECOMBIVAX HB® [hepatitis B vaccine (recombinant)] in Girls and Women 16 Through 23 Years of Age
The safety of GARDASIL when administered concomitantly with RECOMBIVAX HB® [hepatitis B vaccine (recombinant)] was evaluated in an AAHS-controlled study of 1871 girls and women with a mean age of 20.4 years [see Clinical Studies (14.9)]. The race distribution of the study individuals was as follows: 61.6% White; 23.8% Other; 11.9% Black; 1.6% Hispanic (Black and White); 0.8% Asian; and 0.3% American Indian. The rates of systemic and injection-site adverse reactions were similar among girls and women who received concomitant vaccination as compared with those who received GARDASIL or RECOMBIVAX HB [hepatitis B vaccine (recombinant)].
Safety in Concomitant Use with Menactra [Meningococcal (Groups A, C, Y and W-135) Polysaccharide Diphtheria Toxoid Conjugate Vaccine] and Adacel [Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine Adsorbed (Tdap)]
The safety of GARDASIL when administered concomitantly with Menactra [Meningococcal (Groups A, C, Y and W-135) Polysaccharide Diphtheria Toxoid Conjugate Vaccine] and Adacel [Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine Adsorbed (Tdap)] was evaluated in a randomized study of 1040 boys and girls with a mean age of 12.6 years [see Clinical Studies (14.10)]. The race distribution of the study subjects was as follows: 77.7% White; 1.4% Multi-racial; 12.3% Black; 6.8% Hispanic (Black and White); 1.2% Asian; 0.4% American Indian, and 0.2% Indian.
There was an increase in injection-site swelling reported at the injection site for GARDASIL (concomitant = 10.9%, non-concomitant = 6.9%) when GARDASIL was administered concomitantly with Menactra and Adacel as compared to non-concomitant (separated by 1 month) vaccination. The majority of injection-site swelling adverse experiences were reported as being mild to moderate in intensity.
Safety in Women 27 Through 45 Years of Age
The adverse reaction profile in women 27 through 45 years of age was comparable to the profile seen in girls and women 9 through 26 years of age.
Postmarketing Experience
The following adverse events have been spontaneously reported during post-approval use of GARDASIL. Because these events were reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or to establish a causal relationship to vaccine exposure.
- Blood and lymphatic system disorders: Autoimmune hemolytic anemia, idiopathic thrombocytopenic purpura, lymphadenopathy.
- Respiratory, thoracic and mediastinal disorders: Pulmonary embolus.
- Gastrointestinal disorders: Nausea, pancreatitis, vomiting.
- General disorders and administration site conditions: Asthenia, chills, death, fatigue, malaise.
- Immune system disorders: Autoimmune diseases, hypersensitivity reactions including anaphylactic/anaphylactoid reactions, bronchospasm, and urticaria.
- Musculoskeletal and connective tissue disorders: Arthralgia, myalgia.
- Nervous system disorders: Acute disseminated encephalomyelitis, dizziness, Guillain-Barré syndrome, headache, motor neuron disease, paralysis, seizures, syncope (including syncope associated with tonic-clonic movements and other seizure-like activity) sometimes resulting in falling with injury, transverse myelitis.
- Infections and infestations: cellulitis.
- Vascular disorders: Deep venous thrombosis.
Drug Interactions
Use with RECOMBIVAX HB
Results from clinical studies indicate that GARDASIL may be administered concomitantly (at a separate injection site) with RECOMBIVAX HB [hepatitis B vaccine (recombinant)] [see Clinical Studies (14.9)].
Use with Menactra and Adacel
Results from clinical studies indicate that GARDASIL may be administered concomitantly (at a separate injection site) with Menactra [Meningococcal (Groups A, C, Y and W-135) Polysaccharide Diphtheria Toxoid Conjugate Vaccine] and Adacel [Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine Adsorbed (Tdap)].
Use with Hormonal Contraceptives
In clinical studies of 16- through 26-year-old women, 13,912 (GARDASIL N = 6952; AAHS control or saline placebo N = 6960) who had post-Month 7 follow-up used hormonal contraceptives for a total of 33,859 person-years (65.8% of the total follow-up time in the studies).
In one clinical study of 24- through 45-year-old women, 1357 (GARDASIL N = 690; AAHS control N = 667) who had post-Month 7 follow-up used hormonal contraceptives for a total of 3400 person-years (31.5% of the total follow-up time in the study). Use of hormonal contraceptives or lack of use of hormonal contraceptives among study participants did not impair the immune response in the per protocol immunogenicity (PPI) population.
Use with Systemic Immunosuppressive Medications
Immunosuppressive therapies, including irradiation, antimetabolites, alkylating agents, cytotoxic drugs, and corticosteroids (used in greater than physiologic doses), may reduce the immune responses to vaccines
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): B Reproduction studies have been performed in female rats at doses equivalent to the recommended human dose and have revealed no evidence of impaired female fertility or harm to the fetus due to GARDASIL. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human responses, GARDASIL should be used during pregnancy only if clearly needed.
An evaluation of the effect of GARDASIL on embryo-fetal, pre- and postweaning development was conducted using rats. One group of rats was administered GARDASIL twice prior to gestation, during the period of organogenesis (gestation Day 6) and on lactation Day 7. A second group of pregnant rats was administered GARDASIL during the period of organogenesis (gestation Day 6) and on lactation Day 7 only. GARDASIL was administered at 0.5 mL/rat/occasion (120 mcg total protein which is equivalent to the recommended human dose) by intramuscular injection. No adverse effects on mating, fertility, pregnancy, parturition, lactation, embryo-fetal or pre- and postweaning development were observed. There were no vaccine-related fetal malformations or other evidence of teratogenesis noted in this study. In addition, there were no treatment-related effects on developmental signs, behavior, reproductive performance, or fertility of the offspring.
Clinical Studies in Humans
In clinical studies, women underwent urine pregnancy testing prior to administration of each dose of GARDASIL. Women who were found to be pregnant before completion of a 3-dose regimen of GARDASIL were instructed to defer completion of their vaccination regimen until resolution of the pregnancy. GARDASIL is not indicated for women 27 years of age or older. However, safety data in women 16 through 45 years of age was collected, and 3819 women (GARDASIL N = 1894 vs. AAHS control or saline placebo N = 1925) reported at least 1 pregnancy each.
The overall proportions of pregnancies that resulted in an adverse outcome, defined as the combined numbers of spontaneous abortion, late fetal death, and congenital anomaly cases out of the total number of pregnancy outcomes for which an outcome was known (and excluding elective terminations), were 22.6% (446/1973) in women who received GARDASIL and 23.1% (460/1994) in women who received AAHS control or saline placebo.
Overall, 55 and 65 women in the group that received GARDASIL or AAHS control or saline placebo, respectively (2.9% and 3.4% of all women who reported a pregnancy in the respective vaccination groups), experienced a serious adverse reaction during pregnancy. The most common events reported were conditions that can result in Caesarean section (e.g., failure of labor, malpresentation, cephalopelvic disproportion), premature onset of labor (e.g., threatened abortions, premature rupture of membranes), and pregnancy-related medical problems (e.g., pre-eclampsia, hyperemesis). The proportions of pregnant women who experienced such events were comparable between the groups receiving GARDASIL and AAHS control or saline placebo.
There were 45 cases of congenital anomaly in pregnancies that occurred in women who received GARDASIL and 34 cases of congenital anomaly in pregnancies that occurred in women who received AAHS control or saline placebo.
Further sub-analyses were conducted to evaluate pregnancies with estimated onset within 30 days or more than 30 days from administration of a dose of GARDASIL or AAHS control or saline placebo. For pregnancies with estimated onset within 30 days of vaccination, 5 cases of congenital anomaly were observed in the group that received GARDASIL compared to 1 case of congenital anomaly in the group that received AAHS control or saline placebo. The congenital anomalies seen in pregnancies with estimated onset within 30 days of vaccination included pyloric stenosis, congenital megacolon, congenital hydronephrosis, hip dysplasia, and club foot. Conversely, in pregnancies with onset more than 30 days following vaccination, 40 cases of congenital anomaly were observed in the group that received GARDASIL compared with 33 cases of congenital anomaly in the group that received AAHS control or saline placebo.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of HPV Vaccine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of HPV Vaccine during labor and delivery.
Nursing Mothers
Women 16 Through 45 Years of Age
It is not known whether GARDASIL is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when GARDASIL is administered to a nursing woman. GARDASIL or AAHS control were given to a total of 1133 women (vaccine N = 582, AAHS control N = 551) during the relevant Phase III clinical studies.
Overall, 27 and 13 infants of women who received GARDASIL or AAHS control, respectively (representing 4.6% and 2.4% of the total number of women who were breast-feeding during the period in which they received GARDASIL or AAHS control, respectively), experienced a serious adverse reaction.
In a post-hoc analysis of clinical studies, a higher number of breast-feeding infants (n = 7) whose mothers received GARDASIL had acute respiratory illnesses within 30 days post vaccination of the mother as compared to infants (n = 2) whose mothers received AAHS control.
Pediatric Use
Safety and effectiveness have not been established in pediatric patients below 9 years of age.
Geriatic Use
The safety and effectiveness of GARDASIL have not been evaluated in a geriatric population, defined as individuals aged 65 years and over.
Gender
There is no FDA guidance on the use of HPV Vaccine with respect to specific gender populations.
Race
There is no FDA guidance on the use of HPV Vaccine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of HPV Vaccine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of HPV Vaccine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of HPV Vaccine in women of reproductive potentials and males.
Immunocompromised Patients
The immunologic response to GARDASIL may be diminished in immunocompromised individuals
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
Solution
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Y-Site
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Admixture
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Syringe
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
TPN/TNA
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Overdosage
Acute Overdose
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description)
Pharmacology
HPV Vaccine
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
(Description)
Structure
(Description with picture)
Pharmacodynamics
(Description)
Pharmacokinetics
(Description)
Nonclinical Toxicology
(Description)
Clinical Studies
Condition 1
(Description)
Condition 2
(Description)
Condition 3
(Description)
How Supplied
(Description)
Storage
There is limited information regarding HPV Vaccine Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::HPV Vaccine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::HPV Vaccine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
(Patient Counseling Information)
Precautions with Alcohol
Alcohol-HPV Vaccine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding HPV Vaccine Brand Names in the drug label.
Look-Alike Drug Names
- (Paired Confused Name 1a) — (Paired Confused Name 1b)
- (Paired Confused Name 2a) — (Paired Confused Name 2b)
- (Paired Confused Name 3a) — (Paired Confused Name 3b)
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ 1.0 1.1 Brown DR, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM; et al. (2009). "The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16-26 years". J Infect Dis. 199 (7): 926–35. doi:10.1086/597307. PMID 19236279.
- ↑ 2.0 2.1 Wheeler CM, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Perez G; et al. (2009). "The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in sexually active women aged 16-26 years". J Infect Dis. 199 (7): 936–44. doi:10.1086/597309. PMID 19236277.