Granulomatosis with polyangiitis pathophysiology
|
Granulomatosis with polyangiitis Microchapters |
|
Differentiating Granulomatosis with polyangiitis from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Granulomatosis with polyangiitis pathophysiology On the Web |
|
American Roentgen Ray Society Images of Granulomatosis with polyangiitis pathophysiology |
|
Granulomatosis with polyangiitis pathophysiology in the news |
|
Directions to Hospitals Treating Granulomatosis with polyangiitis |
|
Risk calculators and risk factors for Granulomatosis with polyangiitis pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]Ali Poyan Mehr, M.D. [2] Associate Editor(s)-in-Chief: Cafer Zorkun, M.D., Ph.D. [3]Krzysztof Wierzbicki M.D. [4]
Overview
Inflammation with granuloma formation against a nonspecific inflammatory background is the classical tissue abnormality in all organs affected by Wegener's granulomatosis.
Pathogenesis
The pathogenesis of Granulomatosis with polyangiitis is currently unknown. However, several suggestions have been made to identify possible links associated with the disease, such as bacterial infections, failure of B-cells to downregulate, and T cell dysfunction.
Bacterial infections invoke Granulomatosis with polyangiitis by: causing granulocytes to become active, form autoantibodies against microbial antigens and host proteins.[1].
Another possible cause of this disease is the failure of B cells to downregulate ANCA autoimmunity because of CD19 dysregulation at two stages. The first is CD19 naïve B cells, the dysregulation of CD19 B naïve cells may result in B cells to be autoreactive and have the ability to activate themselves. Another stage of dysregulation is CD19 memory B cells, this allows increased sensitive to reactivate B cells.[2]
The last pathogenic cause of ANCA is the dysfunction of T regulator cells (CD4+ CD25+). An imbalance between effector cells and regulatory T cells invokes the development of ANCA. Interleukin 23 causes T cells to become T helper 17 cells that promote Interleukin 17, Interleukin 6, and tumor necrosis factor alpha to produce pro-inflammatory cytokines.
Genetics
The genetic component of Granulomatosis with polyangiitis is not fully known. However, there seems to be a strong correlation between HLA-DPB1 and HLA-DPB2 with Granulomatosis with polyangiitis.[3]
Associated Conditions
Microscopic Pathology
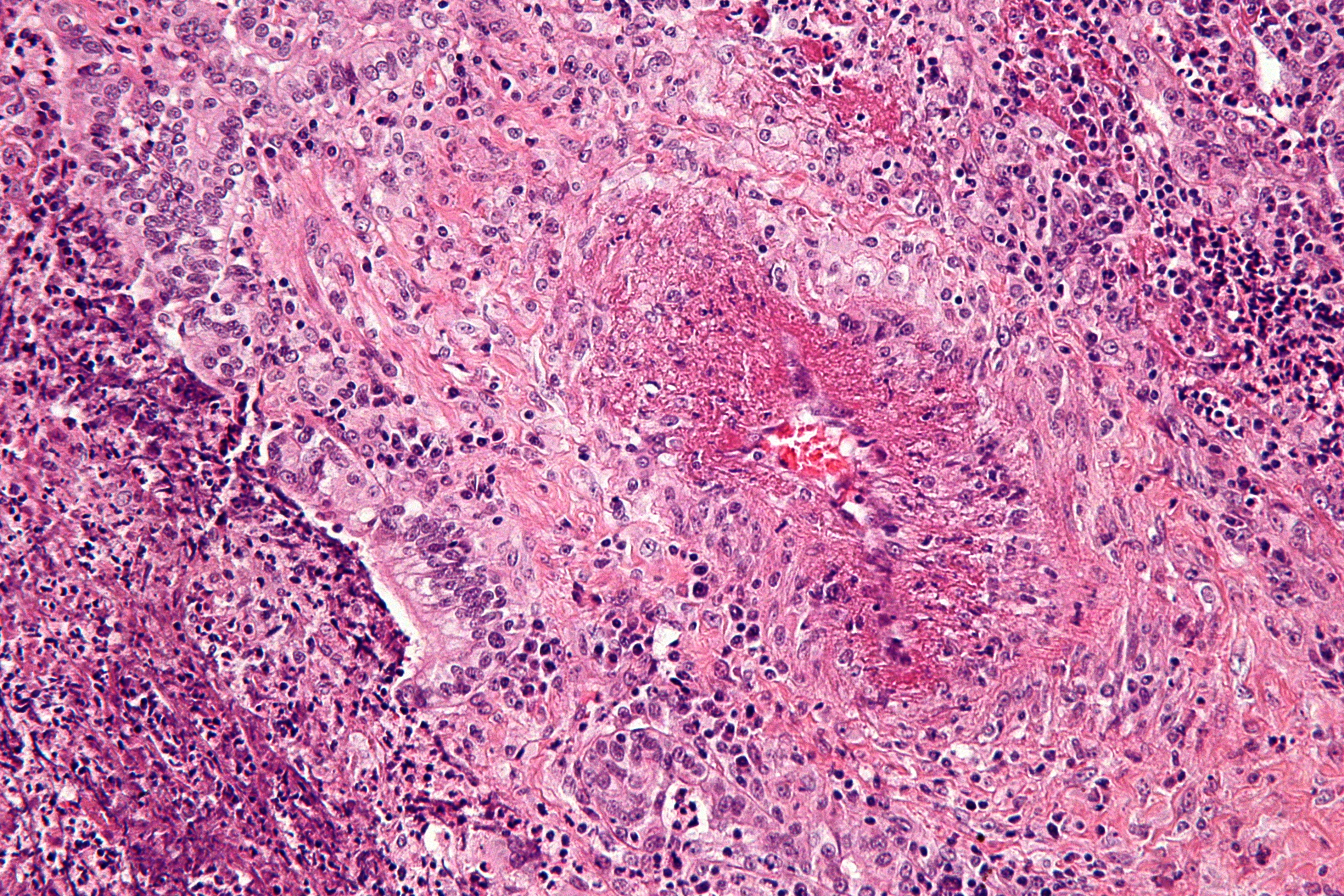
On microscopic histopathology analysis, focal and segmental necrotizing glomerulitis, presence of non-caseating granuloma, necrotizing vasculitis, varied multinucleated giant cells at times are characteristic findings of Granulomatosis with polyangiitis. The following is an image of the microscopic pathology of Granulomatosis with polyangiitis: [4]
Pathophysiology
It is now widely presumed that the anti-neutrophil cytoplasmic antibodies (ANCAs) are responsible for the inflammation in Wegener's. The typical ANCAs in Wegener's are those that react with proteinase 3, an enzyme prevalent in neutrophil granulocytes.[5] This type of ANCA is also known as cANCA, with the cindicating cytoplasmic (in contrast to pANCA, which is perinuclear).
ANCAs activate neutrophils, increase their adherence to endothelium, and lead to their degranulation. This causes extensive damage to the vessel wall, particularly of arterioles.
The exact cause for the production of ANCAs is unknown, although some drugs have been implicated in secondary forms of Wegener's. As with many autoimmune disorders, the cause is probably genetic predisposition combined with molecular mimicry caused by a virus or bacterium.
References
- ↑ Kain R, Exner M, Brandes R, Ziebermayr R, Cunningham D, Alderson CA; et al. (2008). "Molecular mimicry in pauci-immune focal necrotizing glomerulonephritis". Nat Med. 14 (10): 1088–96. doi:10.1038/nm.1874. PMC 2751601. PMID 18836458.
- ↑ Culton DA, Nicholas MW, Bunch DO, Zhen QL, Kepler TB, Dooley MA; et al. (2007). "Similar CD19 dysregulation in two autoantibody-associated autoimmune diseases suggests a shared mechanism of B-cell tolerance loss". J Clin Immunol. 27 (1): 53–68. doi:10.1007/s10875-006-9051-1. PMID 17195045.
- ↑ Xie G, Roshandel D, Sherva R, Monach PA, Lu EY, Kung T; et al. (2013). "Association of granulomatosis with polyangiitis (Wegener's) with HLA-DPB1*04 and SEMA6A gene variants: evidence from genome-wide analysis". Arthritis Rheum. 65 (9): 2457–68. doi:10.1002/art.38036. PMC 4471994. PMID 23740775.
- ↑ Libre pathology. https://librepathology.org/wiki/Granulomatosis_with_polyangiitis Accessed on November 7, 2016
- ↑ van der Woude FJ, Rasmussen N, Lobatto S, Wiik A, Permin H, van Es LA, van der Giessen M, van der Hem GK, The TH. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener's granulomatosis. Lancet1985;1(8426):425-9. PMID 2857806.