Granulomatosis with polyangiitis pathophysiology: Difference between revisions
No edit summary |
|||
| Line 7: | Line 7: | ||
==Pathogenesis== | ==Pathogenesis== | ||
The pathogenesis of Granulomatosis with polyangiitis is currently unknown. However, several suggestions have been made to identify possible links associated with the disease, such as bacterial infections, failure of B-cells to downregulate, and T cell dysfunction. | The pathogenesis of Granulomatosis with polyangiitis is currently unknown. However, several suggestions have been made to identify possible links associated with the disease, such as bacterial infections, failure of B-cells to downregulate, and T cell dysfunction. | ||
Bacterial infections invoke Granulomatosis with polyangiitis by: causing granulocytes to become active, forming autoantibodies against microbial antigens and host proteins.<ref name="pmid18836458">{{cite journal| author=Kain R, Exner M, Brandes R, Ziebermayr R, Cunningham D, Alderson CA et al.| title=Molecular mimicry in pauci-immune focal necrotizing glomerulonephritis. | journal=Nat Med | year= 2008 | volume= 14 | issue= 10 | pages= 1088-96 | pmid=18836458 | doi=10.1038/nm.1874 | pmc=2751601 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18836458 }} </ref>. | === Role of bacterial antigens === | ||
* Bacterial infections invoke Granulomatosis with polyangiitis by: causing granulocytes to become active, forming autoantibodies against microbial antigens and host proteins.<ref name="pmid18836458">{{cite journal| author=Kain R, Exner M, Brandes R, Ziebermayr R, Cunningham D, Alderson CA et al.| title=Molecular mimicry in pauci-immune focal necrotizing glomerulonephritis. | journal=Nat Med | year= 2008 | volume= 14 | issue= 10 | pages= 1088-96 | pmid=18836458 | doi=10.1038/nm.1874 | pmc=2751601 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18836458 }} </ref>. | |||
Another possible cause of this disease is the failure of B cells to downregulate ANCA autoimmunity because of CD19 dysregulation at two stages. The first is CD19 naïve B cells, the dysregulation of CD19 B naïve cells may result in B cells to be autoreactive and have the ability to activate themselves. Another stage of dysregulation is CD19 memory B cells, this allows increased sensitive to reactivate B cells.<ref name="pmid17195045">{{cite journal| author=Culton DA, Nicholas MW, Bunch DO, Zhen QL, Kepler TB, Dooley MA et al.| title=Similar CD19 dysregulation in two autoantibody-associated autoimmune diseases suggests a shared mechanism of B-cell tolerance loss. | journal=J Clin Immunol | year= 2007 | volume= 27 | issue= 1 | pages= 53-68 | pmid=17195045 | doi=10.1007/s10875-006-9051-1 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17195045 }} </ref> | === Role of auto-immunity === | ||
* Another possible cause of this disease is the failure of B cells to downregulate ANCA autoimmunity because of CD19 dysregulation at two stages. | |||
* The first is CD19 naïve B cells, the dysregulation of CD19 B naïve cells may result in B cells to be autoreactive and have the ability to activate themselves. | |||
* Another stage of dysregulation is CD19 memory B cells, this allows increased sensitive to reactivate B cells.<ref name="pmid17195045">{{cite journal| author=Culton DA, Nicholas MW, Bunch DO, Zhen QL, Kepler TB, Dooley MA et al.| title=Similar CD19 dysregulation in two autoantibody-associated autoimmune diseases suggests a shared mechanism of B-cell tolerance loss. | journal=J Clin Immunol | year= 2007 | volume= 27 | issue= 1 | pages= 53-68 | pmid=17195045 | doi=10.1007/s10875-006-9051-1 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17195045 }} </ref> | |||
The last pathogenic cause of ANCA is the dysfunction of T regulator cells (CD4+ CD25+). An imbalance between effector cells and regulatory T cells invokes the development of ANCA. The presence of ANCA, induces Interleukin 23 (Il-23) to produce T helper 17 cells. The production of T helper 17 cells promotes the production of Il-17, Il-6, and tumor necrosis factor alpha (TNF-α) which invokes the inflammation of cytokines. <ref name="pmid27596099">{{cite journal| author=Noone D, Hebert D, Licht C| title=Pathogenesis and treatment of ANCA-associated vasculitis-a role for complement. | journal=Pediatr Nephrol | year= 2016 | volume= | issue= | pages= | pmid=27596099 | doi=10.1007/s00467-016-3475-5 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=27596099 }}</ref> | === Dysfunction of T regulator cells === | ||
* The last pathogenic cause of ANCA is the dysfunction of T regulator cells (CD4+ CD25+). An imbalance between effector cells and regulatory T cells invokes the development of ANCA. | |||
* The presence of ANCA, induces Interleukin 23 (Il-23) to produce T helper 17 cells. The production of T helper 17 cells promotes the production of Il-17, Il-6, and tumor necrosis factor alpha (TNF-α) which invokes the inflammation of cytokines. <ref name="pmid27596099">{{cite journal| author=Noone D, Hebert D, Licht C| title=Pathogenesis and treatment of ANCA-associated vasculitis-a role for complement. | journal=Pediatr Nephrol | year= 2016 | volume= | issue= | pages= | pmid=27596099 | doi=10.1007/s00467-016-3475-5 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=27596099 }}</ref> | |||
The inflammation | === Neutrophil activation === | ||
* The inflammation due to cytokines and the presence of ANCA allows neutrophils to bind TNFα that are actively present on the endothelium, ANCA can cause neutrophils to become active. | |||
* This is due to Fragment secondary antibodies of ANCA that bind to proteinase 3 or myeloperoxidase. | |||
* The Fragment crystallizable (Fc) binds to the Fragment crystallizable- gamma receptor (Fc-γ) which allows neutrophils to become active. With this activation, the endothelium becomes destroyed. This is due to degranulation and reactive oxygen species. <ref name="pmid16126939">{{cite journal| author=van Rossum AP, Limburg PC, Kallenberg CG| title=Activation, apoptosis, and clearance of neutrophils in Wegener's granulomatosis. | journal=Ann N Y Acad Sci | year= 2005 | volume= 1051 | issue= | pages= 1-11 | pmid=16126939 | doi=10.1196/annals.1361.041 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16126939 }}</ref> | |||
==Genetics== | ==Genetics== | ||
| Line 27: | Line 37: | ||
==Gross Pathology== | ==Gross Pathology== | ||
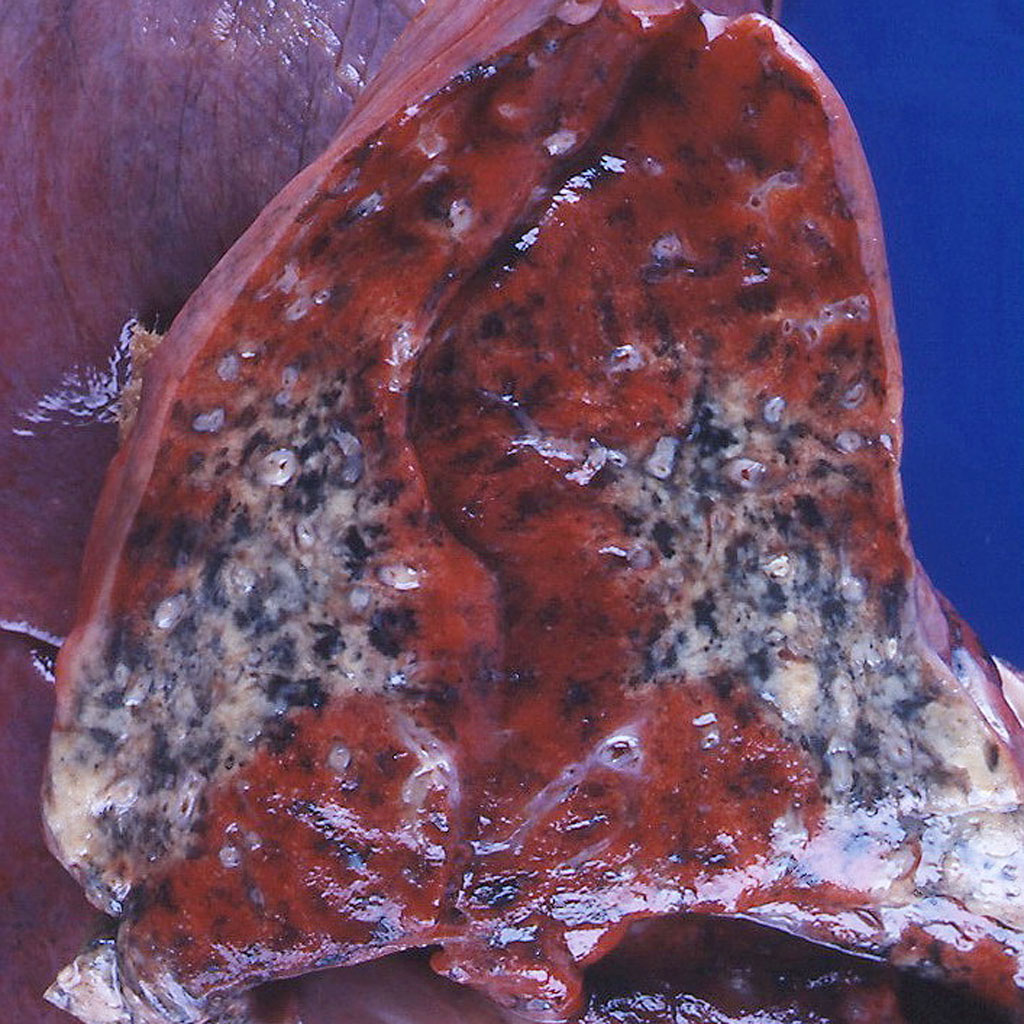
On gross pathology, ulceration, necrosis of bone and cartilage, and vascular necrosis are non specific findings in Granulomatosis with polyangiitis.<ref name="pmid13560836">{{cite journal| author=WALTON EW| title=Giant-cell granuloma of the respiratory tract (Wegener's granulomatosis). | journal=Br Med J | year= 1958 | volume= 2 | issue= 5091 | pages= 265-70 | pmid=13560836 | doi= | pmc=2026251 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=13560836 }}</ref> The following is an image of the gross pathology of Granulomatosis with polyangiitis:<ref name="Gross pathology of Granulomatosis with polyangiitis"> Case courtesy of Dr. Yale Rosen. https://radiopaedia.org/cases/8950 Accessed on November 9, 2016 </ref> | On gross pathology, ulceration, necrosis of bone and cartilage, and vascular necrosis are non specific findings in Granulomatosis with polyangiitis.<ref name="pmid13560836">{{cite journal| author=WALTON EW| title=Giant-cell granuloma of the respiratory tract (Wegener's granulomatosis). | journal=Br Med J | year= 1958 | volume= 2 | issue= 5091 | pages= 265-70 | pmid=13560836 | doi= | pmc=2026251 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=13560836 }}</ref> The following is an image of the gross pathology of Granulomatosis with polyangiitis:<ref name="Gross pathology of Granulomatosis with polyangiitis">Case courtesy of Dr. Yale Rosen. https://radiopaedia.org/cases/8950 Accessed on November 9, 2016 </ref> | ||
<gallery> | <gallery> | ||
| Line 36: | Line 46: | ||
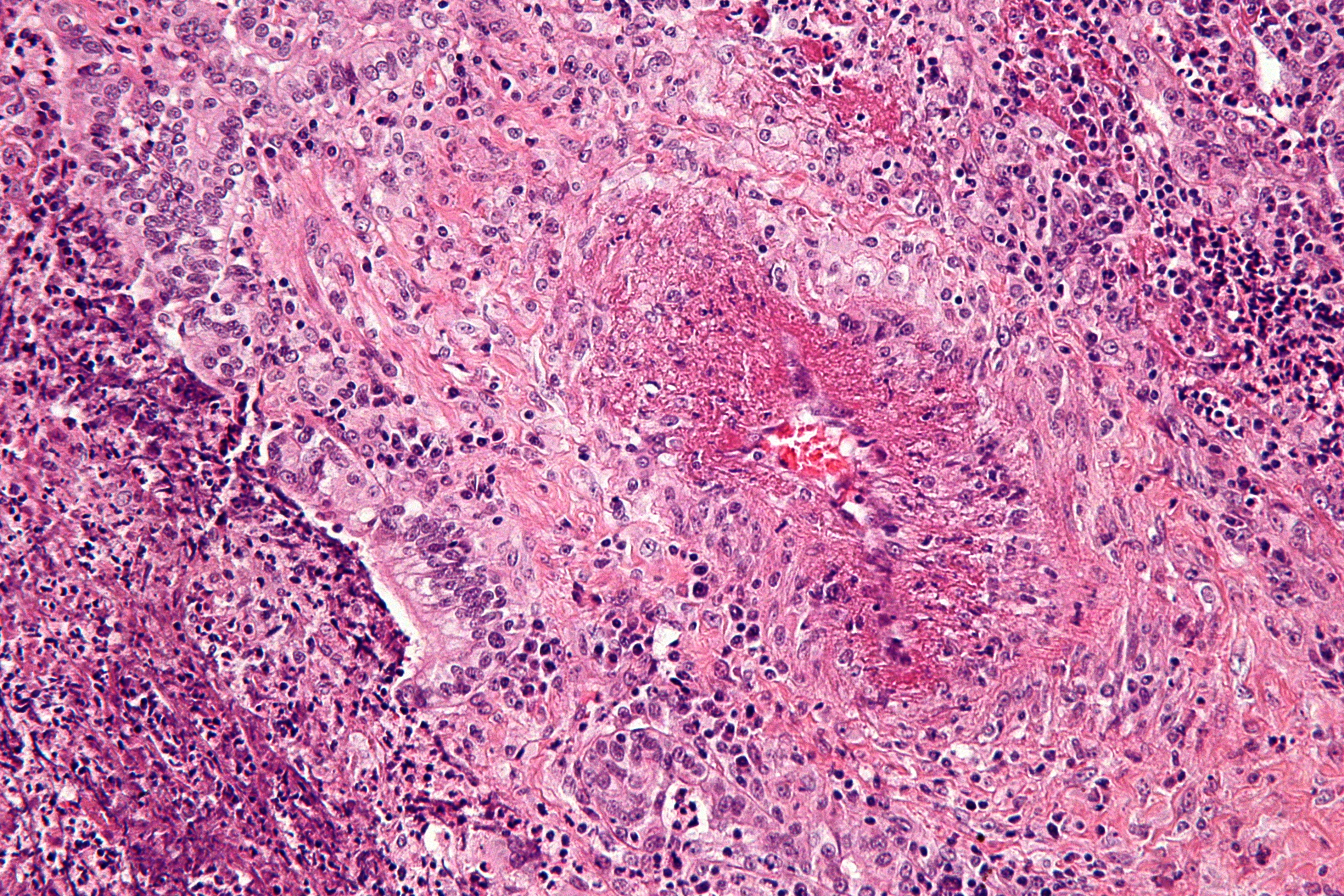
On microscopic histopathology analysis, focal and segmental necrotizing glomerulitis, presence of non-caseating granuloma, necrotizing vasculitis, varied multinucleated giant cells at times are characteristic findings of Granulomatosis with polyangiitis.<ref name="pmid1975173">{{cite journal| author=Lie JT| title=Illustrated histopathologic classification criteria for selected vasculitis syndromes. American College of Rheumatology Subcommittee on Classification of Vasculitis. | journal=Arthritis Rheum | year= 1990 | volume= 33 | issue= 8 | pages= 1074-87 | pmid=1975173 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=1975173 }} </ref> | On microscopic histopathology analysis, focal and segmental necrotizing glomerulitis, presence of non-caseating granuloma, necrotizing vasculitis, varied multinucleated giant cells at times are characteristic findings of Granulomatosis with polyangiitis.<ref name="pmid1975173">{{cite journal| author=Lie JT| title=Illustrated histopathologic classification criteria for selected vasculitis syndromes. American College of Rheumatology Subcommittee on Classification of Vasculitis. | journal=Arthritis Rheum | year= 1990 | volume= 33 | issue= 8 | pages= 1074-87 | pmid=1975173 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=1975173 }} </ref> | ||
<ref name="pmid25076302">{{cite journal| author=Muller K, Lin JH| title=Orbital granulomatosis with polyangiitis (Wegener granulomatosis): clinical and pathologic findings. | journal=Arch Pathol Lab Med | year= 2014 | volume= 138 | issue= 8 | pages= 1110-4 | pmid=25076302 | doi=10.5858/arpa.2013-0006-RS | pmc=4140401 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=25076302 }} </ref> | <ref name="pmid25076302">{{cite journal| author=Muller K, Lin JH| title=Orbital granulomatosis with polyangiitis (Wegener granulomatosis): clinical and pathologic findings. | journal=Arch Pathol Lab Med | year= 2014 | volume= 138 | issue= 8 | pages= 1110-4 | pmid=25076302 | doi=10.5858/arpa.2013-0006-RS | pmc=4140401 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=25076302 }} </ref> | ||
The following image is the microscopic pathology of Granulomatosis with polyangiitis: <ref name="Granulomatosis with polyangiitis"> Libre pathology. https://librepathology.org/wiki/Granulomatosis_with_polyangiitis Accessed on November 7, 2016</ref> | The following image is the microscopic pathology of Granulomatosis with polyangiitis: <ref name="Granulomatosis with polyangiitis">Libre pathology. https://librepathology.org/wiki/Granulomatosis_with_polyangiitis Accessed on November 7, 2016</ref> | ||
<gallery> | <gallery> | ||
Revision as of 13:55, 27 March 2018
|
Granulomatosis with polyangiitis Microchapters |
|
Differentiating Granulomatosis with polyangiitis from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Granulomatosis with polyangiitis pathophysiology On the Web |
|
American Roentgen Ray Society Images of Granulomatosis with polyangiitis pathophysiology |
|
Granulomatosis with polyangiitis pathophysiology in the news |
|
Directions to Hospitals Treating Granulomatosis with polyangiitis |
|
Risk calculators and risk factors for Granulomatosis with polyangiitis pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]Ali Poyan Mehr, M.D. [2] Associate Editor(s)-in-Chief: Krzysztof Wierzbicki M.D. [3]Cafer Zorkun, M.D., Ph.D. [4]
Overview
The pathogenesis of Granulomatosis with polyangiitis is currently unknown. However, several hypothesizes have been made to identify possible links associated with this disease, such as bacterial infections, failure of B-cells to downregulate, and T cell dysfunction. The genetic component of Granulomatosis with polyangiitis is not fully known. However, there seems to be a strong correlation between HLA-DPB1 and HLA-DPB2 with Granulomatosis with polyangiitis.[1]
Pathogenesis
The pathogenesis of Granulomatosis with polyangiitis is currently unknown. However, several suggestions have been made to identify possible links associated with the disease, such as bacterial infections, failure of B-cells to downregulate, and T cell dysfunction.
Role of bacterial antigens
- Bacterial infections invoke Granulomatosis with polyangiitis by: causing granulocytes to become active, forming autoantibodies against microbial antigens and host proteins.[2].
Role of auto-immunity
- Another possible cause of this disease is the failure of B cells to downregulate ANCA autoimmunity because of CD19 dysregulation at two stages.
- The first is CD19 naïve B cells, the dysregulation of CD19 B naïve cells may result in B cells to be autoreactive and have the ability to activate themselves.
- Another stage of dysregulation is CD19 memory B cells, this allows increased sensitive to reactivate B cells.[3]
Dysfunction of T regulator cells
- The last pathogenic cause of ANCA is the dysfunction of T regulator cells (CD4+ CD25+). An imbalance between effector cells and regulatory T cells invokes the development of ANCA.
- The presence of ANCA, induces Interleukin 23 (Il-23) to produce T helper 17 cells. The production of T helper 17 cells promotes the production of Il-17, Il-6, and tumor necrosis factor alpha (TNF-α) which invokes the inflammation of cytokines. [4]
Neutrophil activation
- The inflammation due to cytokines and the presence of ANCA allows neutrophils to bind TNFα that are actively present on the endothelium, ANCA can cause neutrophils to become active.
- This is due to Fragment secondary antibodies of ANCA that bind to proteinase 3 or myeloperoxidase.
- The Fragment crystallizable (Fc) binds to the Fragment crystallizable- gamma receptor (Fc-γ) which allows neutrophils to become active. With this activation, the endothelium becomes destroyed. This is due to degranulation and reactive oxygen species. [5]
Genetics
The genetic component of Granulomatosis with polyangiitis is not fully known. However, there seems to be a strong correlation between HLA-DPB1 and HLA-DPB2 with Granulomatosis with polyangiitis.[1] The genetic inheritance of Granulomatosis with polyangiitis is not common.[6]
Associated Conditions
The following conditions are associated with Granulomatosis with polyangiitis:
Gross Pathology
On gross pathology, ulceration, necrosis of bone and cartilage, and vascular necrosis are non specific findings in Granulomatosis with polyangiitis.[9] The following is an image of the gross pathology of Granulomatosis with polyangiitis:[10]
Microscopic Pathology
On microscopic histopathology analysis, focal and segmental necrotizing glomerulitis, presence of non-caseating granuloma, necrotizing vasculitis, varied multinucleated giant cells at times are characteristic findings of Granulomatosis with polyangiitis.[11] [12] The following image is the microscopic pathology of Granulomatosis with polyangiitis: [13]
References
- ↑ 1.0 1.1 Xie G, Roshandel D, Sherva R, Monach PA, Lu EY, Kung T; et al. (2013). "Association of granulomatosis with polyangiitis (Wegener's) with HLA-DPB1*04 and SEMA6A gene variants: evidence from genome-wide analysis". Arthritis Rheum. 65 (9): 2457–68. doi:10.1002/art.38036. PMC 4471994. PMID 23740775.
- ↑ Kain R, Exner M, Brandes R, Ziebermayr R, Cunningham D, Alderson CA; et al. (2008). "Molecular mimicry in pauci-immune focal necrotizing glomerulonephritis". Nat Med. 14 (10): 1088–96. doi:10.1038/nm.1874. PMC 2751601. PMID 18836458.
- ↑ Culton DA, Nicholas MW, Bunch DO, Zhen QL, Kepler TB, Dooley MA; et al. (2007). "Similar CD19 dysregulation in two autoantibody-associated autoimmune diseases suggests a shared mechanism of B-cell tolerance loss". J Clin Immunol. 27 (1): 53–68. doi:10.1007/s10875-006-9051-1. PMID 17195045.
- ↑ Noone D, Hebert D, Licht C (2016). "Pathogenesis and treatment of ANCA-associated vasculitis-a role for complement". Pediatr Nephrol. ( ): . doi:10.1007/s00467-016-3475-5. PMID 27596099.
- ↑ van Rossum AP, Limburg PC, Kallenberg CG (2005). "Activation, apoptosis, and clearance of neutrophils in Wegener's granulomatosis". Ann N Y Acad Sci. 1051 ( ): 1–11. doi:10.1196/annals.1361.041. PMID 16126939.
- ↑ Knight A, Sandin S, Askling J (2008). "Risks and relative risks of Wegener's granulomatosis among close relatives of patients with the disease". Arthritis Rheum. 58 (1): 302–7. doi:10.1002/art.23157. PMID 18163522.
- ↑ Langlois V, Lesourd A, Girszyn N, Ménard JF, Levesque H, Caron F; et al. (2016). "Antineutrophil Cytoplasmic Antibodies Associated With Infective Endocarditis". Medicine (Baltimore). 95 (3): e2564. doi:10.1097/MD.0000000000002564. PMC 4998285. PMID 26817911.
- ↑ Kalluri R, Meyers K, Mogyorosi A, Madaio MP, Neilson EG (1997). "Goodpasture syndrome involving overlap with Wegener's granulomatosis and anti-glomerular basement membrane disease". J Am Soc Nephrol. 8 (11): 1795–800. PMID 9355084.
- ↑ WALTON EW (1958). "Giant-cell granuloma of the respiratory tract (Wegener's granulomatosis)". Br Med J. 2 (5091): 265–70. PMC 2026251. PMID 13560836.
- ↑ Case courtesy of Dr. Yale Rosen. https://radiopaedia.org/cases/8950 Accessed on November 9, 2016
- ↑ Lie JT (1990). "Illustrated histopathologic classification criteria for selected vasculitis syndromes. American College of Rheumatology Subcommittee on Classification of Vasculitis". Arthritis Rheum. 33 (8): 1074–87. PMID 1975173.
- ↑ Muller K, Lin JH (2014). "Orbital granulomatosis with polyangiitis (Wegener granulomatosis): clinical and pathologic findings". Arch Pathol Lab Med. 138 (8): 1110–4. doi:10.5858/arpa.2013-0006-RS. PMC 4140401. PMID 25076302.
- ↑ Libre pathology. https://librepathology.org/wiki/Granulomatosis_with_polyangiitis Accessed on November 7, 2016