Garenoxacin
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
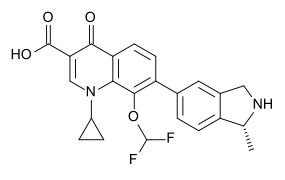
| Formula | C23H20F2N2O4 |
| Molar mass | 426.412 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
|
WikiDoc Resources for Garenoxacin |
|
Articles |
|---|
|
Most recent articles on Garenoxacin Most cited articles on Garenoxacin |
|
Media |
|
Powerpoint slides on Garenoxacin |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Garenoxacin at Clinical Trials.gov Clinical Trials on Garenoxacin at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Garenoxacin
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Garenoxacin Discussion groups on Garenoxacin Patient Handouts on Garenoxacin Directions to Hospitals Treating Garenoxacin Risk calculators and risk factors for Garenoxacin
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Garenoxacin |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Garenoxacin (INN) is a quinolone antibiotic for the treatment of Gram-positive and Gram-negative bacterial infections.
Garenoxacin was discovered by Toyama Chemical Co., Ltd. of Tokyo, Japan, and is currently being marketed in Japan under the tradename Geninax. Schering-Plough holds worldwide rights for garenoxacin, except for Japan, South Korea, and China.
On February 13, 2006, Schering-Plough announced that the United States Food and Drug Administration has accepted the New Drug Application (NDA) for garenoxacin, and has been granted a 10-month review.[1]
Schering-Plough later withdrew its application to the United States Food and Drug Administration, FDA, (August 20, 2006) for approval of the antibiotic Garenoxacin.[2]
The European Medicines Agency (EMEA) had also been formally notified by Schering-Plough Europe (July 28 2007) of its decision to withdraw the application for a centralized marketing authorization for garenoxacin as well.[3][4][5] Based on the CHMP review of the data regarding safety and efficacy (risk/benefit), the CHMP considered the application for garenoxacin to be unapprovable.[6]
References
- ↑ "Drugs.com, Schering-Plough Reports Garenoxacin NDA Accepted for FDA Review". Retrieved 2008-03-25.
- ↑ http://www.fiercebiotech.com/story/schering-plough-pulls-its-garenoxacin-app/2006-08-21
- ↑ http://www.medicalnewstoday.com/articles/78052.php
- ↑ http://www.emea.europa.eu/humandocs/PDFs/EPAR/garenoxacinmesylate/34117407en.pdf
- ↑ http://www.emea.europa.eu/humandocs/PDFs/EPAR/garenoxacinmesylate/H-747-WAR.pdf
- ↑ http://www.emea.europa.eu/humandocs/PDFs/EPAR/garenoxacinmesylate/H-747-WAR.pdf
- Pages with script errors
- Articles with changed CASNo identifier
- Articles with changed ChemSpider identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles with changed InChI identifier
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without DrugBank identifier
- Drugs with no legal status
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Quinolone antibiotics
- Phenol ethers
- Drug