Fungus
| Fungi Fossil range: Early Devonian - Recent (but see text) | ||||||
|---|---|---|---|---|---|---|
 Clockwise from top left: Amanita muscaria, a basidiomycete; Sarcoscypha coccinea, an ascomycete; black bread mold, a zygomycete; a chytrid; a Penicillium conidiophore.
| ||||||
| Scientific classification | ||||||
| ||||||
| Subkingdoms/Phyla | ||||||
|
Dikarya (inc. Deuteromycota) |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
A fungus (Template:PronEng) is a eukaryotic organism that is a member of the kingdom Fungi (Template:PronEng).[2] The fungi are heterotrophic organisms possessing a chitinous cell wall. The majority of species grow as multicellular filaments called hyphae forming a mycelium; some fungal species also grow as single cells. Sexual and asexual reproduction of the fungi is commonly via spores, often produced on specialized structures or in fruiting bodies. Some species have lost the ability to form specialized reproductive structures, and propagate solely by vegetative growth. Yeasts, molds, and mushrooms are examples of fungi. The fungi are a monophyletic group that is phylogenetically clearly distinct from the morphologically similar slime molds (myxomycetes) and water molds (oomycetes). The fungi are more closely related to animals than plants, yet the discipline of biology devoted to the study of fungi, known as mycology, often falls under a branch of botany.
Occurring worldwide, most fungi are largely invisible to the naked eye, living for the most part in soil, dead matter, and as symbionts of plants, animals, or other fungi. They perform an essential role in all ecosystems in decomposing organic matter and are indispensable in nutrient cycling and exchange. Some fungi become noticeable when fruiting, either as mushrooms or molds. Many fungal species have long been used as a direct source of food, such as mushrooms and truffles and in fermentation of various food products, such as wine, beer, and soy sauce. More recently, fungi are being used as sources for antibiotics used in medicine and various enzymes, such as cellulases, pectinases, and proteases, important for industrial use or as active ingredients of detergents. Many fungi produce bioactive compounds called mycotoxins, such as alkaloids and polyketides that are toxic to animals including humans. Some fungi are used recreationally or in traditional ceremonies as a source of psychotropic compounds. Several species of the fungi are significant pathogens of humans and other animals, and losses due to diseases of crops (e.g., rice blast disease) or food spoilage caused by fungi can have a large impact on human food supply and local economies.
Etymology and definition
The English word fungus is directly adopted from the Latin fungus, meaning "mushroom", used in Horace and Pliny.[3] This in turn is derived from the Greek word sphongos/σφογγος ("sponge"), referring to the macroscopic structures and morphology of some mushrooms and molds and also used in other languages (e.g., the German Schwamm ("sponge") or Schwammerl for some types of mushroom).
Diversity
Fungi have a worldwide distribution, and grow in a wide range of habitats, including deserts. Most fungi grow in terrestrial environments, but several species occur only in aquatic habitats. Fungi along with bacteria are the primary decomposers of organic matter in most if not all terrestrial ecosystems worldwide. Based on observations of the ratio of the number of fungal species to the number of plant species in some environments, the fungal kingdom has been estimated to contain about 1.5 million species. [4] Around 70,000 fungal species have been formally described by taxonomists, but the true dimension of fungal diversity is still unknown. [5] Most fungi grow as thread-like filaments called hyphae, which form a mycelium, while others grow as single cells. [6][7] Until recently many fungal species were described based mainly on morphological characteristics, such as the size and shape of spores or fruiting structures, and biological species concepts; the application of molecular tools, such as DNA sequencing, to study fungal diversity has greatly enhanced the resolution and added robustness to estimates of diversity within various taxonomic groups.[8]
Importance for human use
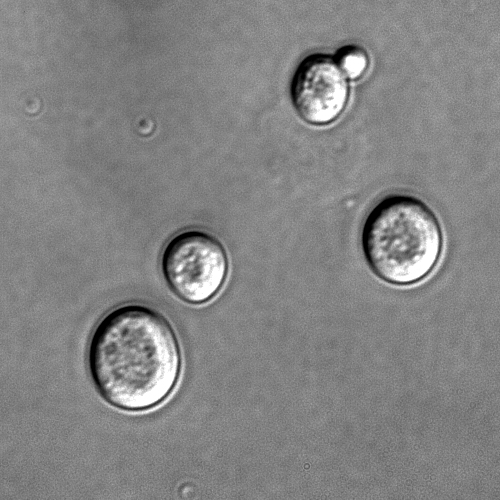
Human use of fungi for food preparation or preservation and other purposes is extensive and has a long history: yeasts are required for fermentation of beer, wine [9] and bread, some other fungal species are used in the production of soy sauce and tempeh. Mushroom farming and mushroom gathering are large industries in many countries. Many fungi are producers of antibiotics, including β-lactam antibiotics such as penicillin and cephalosporin.[10] Widespread use of these antibiotics for the treatment of bacterial diseases, such as tuberculosis, syphilis, leprosy, and many others began in the early 20th century and continues to play a major part in anti-bacterial chemotherapy. The study of the historical uses and sociological impact of fungi is known as ethnomycology.
Cultured foods
Baker's yeast or Saccharomyces cerevisiae, a single-cell fungus, is used in the baking of bread and other wheat-based products, such as pizza and dumplings.[11] Several yeast species of the genus Saccharomyces are also used in the production of alcoholic beverages through fermentation.[12] Mycelial fungi, such as the shoyu koji mold (Aspergillus oryzae), are used in the brewing of Shoyu (soy sauce) and preparation of tempeh.[13] Quorn is a high-protein product made from the mold, Fusarium venenatum, and is used in vegetarian cooking.
Other human uses
Fungi are also used extensively to produce industrial chemicals like lactic acid, antibiotics and even to make stonewashed jeans.[14] Several fungal species are ingested for their psychedelic properties, both recreationally and religiously (see main article, Psilocybin mushrooms).
Mycotoxins
Many fungi produce compounds with biological activity. Several of these compounds are toxic and are therefore called mycotoxins, referring to their fungal origin and toxic activity. Of particular relevance to humans are those mycotoxins that are produced by moulds causing food spoilage and poisonous mushrooms (see below). Particularly infamous are the aflatoxins, which are insidious liver toxins and highly carcinogenic metabolites produced by Aspergillus species often growing in or on grains and nuts consumed by humans, and the lethal amatoxins produced by mushrooms of the genus Amanita. Other notable mycotoxins include ochratoxins, patulin, ergot alkaloids, and trichothecenes and fumonisins, all of which have significant impact on human food supplies or animal livestock. [15]
Mycotoxins belong to the group of secondary metabolites (or natural products). Originally, this group of compounds had been thought to be mere byproducts of primary metabolism, hence the name "secondary" metabolites. However, recent research has shown the existence of biochemical pathways solely for the purpose of producing mycotoxins and other natural products in fungi. [16] Mycotoxins provide a number of fitness benefits to the fungi that produce them in terms of physiological adaptation, competition with other microbes and fungi, and protection from fungivory. [17][18] These fitness benefits and the existence of dedicated biosynthetic pathways for mycotoxin production suggest that the mycotoxins are important for fungal persistence and survival.
Edible and poisonous fungi



Some of the best known types of fungi are the edible and the poisonous mushrooms. Many species are commercially raised, but others must be harvested from the wild. Agaricus bisporus, sold as button mushrooms when small or Portobello mushrooms when larger, are the most commonly eaten species, used in salads, soups, and many other dishes. Many Asian fungi are commercially grown and have gained in popularity in the West. They are often available fresh in grocery stores and markets, including straw mushrooms (Volvariella volvacea), oyster mushrooms (Pleurotus ostreatus), shiitakes (Lentinula edodes), and enokitake (Flammulina spp.).
There are many more mushroom species that are harvested from the wild for personal consumption or commercial sale. Milk mushrooms, morels, chanterelles, truffles, black trumpets, and porcini mushrooms (Boletus edulis) (also known as king boletes) all demand a high price on the market. They are often used in gourmet dishes.
For certain types of cheeses, it is also a common practice to inoculate milk curds with fungal spores to foment the growth of specific species of mold that impart a unique flavor and texture to the cheese. This accounts for the blue colour in cheeses such as Stilton or Roquefort which is created using Penicillium roqueforti spores.[19] Molds used in cheese production are usually non-toxic and are thus safe for human consumption; however, mycotoxins (e.g., aflatoxins, roquefortine C, patulin, or others) may accumulate due to fungal spoilage during cheese ripening or storage.[20]
Many mushroom species are toxic to humans, with toxicities ranging from slight digestive problems or allergic reactions as well as hallucinations to severe organ failures and death. Some of the most deadly mushrooms belong to the genera Inocybe, Cortinarius, and most infamously, Amanita. The latter genus includes the destroying angel (A. virosa) and the death cap (A. phalloides), the most common cause of deadly mushroom poisoning. [21] The false morel (Gyromitra esculenta) is considered a delicacy by some when cooked, yet can be highly toxic when eaten raw. [22] Tricholoma equestre was considered edible until being implicated in some serious poisonings causing rhabdomyolysis. [23]
Fly agaric mushrooms (A. muscaria) also cause occasional poisonings, mostly as a result of ingestion for use as a recreational drug for its hallucinogenic properties. Historically Fly agaric was used by Celtic Druids in Northern Europe and the Koryak people of north-eastern Siberia for religious or shamanic purposes.[24] It is difficult to identify a safe mushroom without proper training and knowledge, thus it is often advised to assume that a mushroom in the wild is poisonous and not to consume it.
Fungi in the biological control of pests
In agricultural settings, fungi that actively compete for nutrients and space with, and eventually prevail over, pathogenic microorganisms, such as bacteria or other fungi, via the competitive exclusion principle,[25] or are parasites of these pathogens, may be beneficial agents for human use. For example, some fungi may be used to suppress growth or eliminate harmful plant pathogens, such as insects, mites, weeds, nematodes and other fungi that cause diseases of important crop plants.[26] This has generated strong interest in the use and practical application of these fungi for the biological control of these agricultural pests. Entomopathogenic fungi can be used as biopesticides, as they actively kill insects.[27] Examples of fungi that have been used as biological insecticides are Beauveria bassiana, Metarhizium anisopliae, Hirsutella spp, Paecilomyces spp, and Verticillium lecanii.[28] [29] Endophytic fungi of grasses of the genus Neotyphodium, such as N. coenophialum produce alkaloids that are toxic to a range of invertebrate and vertebrate herbivores. These alkaloids protect the infected grass plants from herbivory, but some endophyte alkaloids can cause poisoning of grazing animals, such as cattle and sheep. [30] Infection of grass cultivars of turf or forage grasses with isolates of the grass endophytes that produce only specific alkaloids to improve grass hardiness and resistance to herbivores such as insects, while being non-toxic to livestock, is being used in grass breeding programs.[31]
Bioremediation
Certain fungi, in particular 'white rot' fungi, can degrade insecticides, herbicides, pentachlorophenol, creosote, coal tars, and heavy fuels and turn them into carbon dioxide, water, and basic elements.[32] Research has recently discovered that fungi can be used to lock uranium into mineral form.[33]
Ecology

Although often inconspicuous, fungi occur in every environment on Earth and play very important roles in most ecosystems. Along with bacteria, fungi are the major decomposers in most terrestrial (and some aquatic) ecosystems, and therefore play a critical role in biogeochemical cycles and in many food webs. As decomposers, they play an indispensable role in nutrient cycling, especially as saprotrophs and symbionts, degrading organic matter to inorganic molecules, which can then re-enter anabolic metabolic pathways in plants or other organisms.[34][35]
Symbiosis
Many fungi have important symbiotic relationships with organisms from most if not all Kingdoms.[36][37][38] These interactions can be mutualistic or antagonistic in nature, or in case of commensal fungi are of no apparent benefit or detriment to the host. [39][40][41]
With plants
Mycorrhizal symbiosis between plants and fungi is one of the most well-known plant-fungus associations and is of significant importance for plant growth and persistence in many ecosystems; over 90% of all plant species engage in some kind of mycorrhizal relationship with fungi and are dependent upon this relationship for survival.[42][43][44] The mycorrhizal symbiosis is ancient, dating to at least 400 million years ago.[45] It often increases the plant's uptake of inorganic compounds, such as nitrate and phosphate from soils having low concentrations of these key plant nutrients. In some mycorrhizal associations, the fungal partners may mediate plant-to-plant transfer of carbohydrates and other nutrients. Such mycorrhizal communities are called "common mycorrhizal networks". [46]
Lichens are formed by a symbiotic relationship between algae or cyanobacteria (referred to in lichens as "photobionts") and fungi (mostly various species of ascomycetes and a few basidiomycetes), in which individual photobiont cells are embedded in a tissue formed by the fungus.[47] As in mycorrhizas, the photobiont provides sugars and other carbohydrates, while the fungus provides minerals and water. The functions of both symbiotic organisms are so closely intertwined that they function almost as a single organism.
With insects
Many insects also engage in mutualistic relationships with various types of fungi. Several groups of ants cultivate fungi in the order Agaricales as their primary food source, while ambrosia beetles cultivate various species of fungi in the bark of trees that they infest.[48] Termites on the African Savannah are also known to cultivate fungi.[49]
As pathogens and parasites
However, many fungi are parasites on plants, animals (including humans), and other fungi. Serious fungal pathogens of many cultivated plants causing extensive damage and losses to agriculture and forestry include the rice blast fungus Magnaporthe oryzae,[50] tree pathogens such as Ophiostoma ulmi and Ophiostoma novo-ulmi causing Dutch elm disease,[51] and Cryphonectria parasitica responsible for chestnut blight, [52] and plant-pathogenic fungi in the genera Fusarium, Ustilago, Alternaria, and Cochliobolus; [40] fungi with the potential to cause serious human diseases, especially in persons with immuno-deficiencies, are in the genera Aspergillus, Candida, Cryptoccocus,[53][41][54] Histoplasma,[55] and Pneumocystis. [56] Several pathogenic fungi are also responsible for relatively minor human diseases, such as athlete’s foot and ringworm. Some fungi are predators of nematodes, which they capture using an array of specialized structures, such as constricting rings or adhesive nets.[57]
Nutrition and possible autotrophy
Growth of fungi as hyphae on or in solid substrates or single cells in aquatic environments is adapted to efficient extraction of nutrients from these environments, because these growth forms have high surface area to volume ratios. These adaptations in morphology are complemented by hydrolytic enzymes secreted into the environment for digestion of large organic molecules, such as polysaccharides, proteins, lipids, and other organic substrates into smaller molecules. [58][59][60] These molecules are then absorbed as nutrients into the fungal cells.
Traditionally, the fungi are considered heterotrophs, organisms that rely solely on carbon fixed by other organisms for metabolism. Fungi have evolved a remarkable metabolic versatility that allows many of them to use a large variety of organic substrates for growth, including simple compounds as nitrate, ammonia, acetate, or ethanol.[61] [62] Recent research raises the possibility that some fungi utilize the pigment melanin to extract energy from ionizing radiation, such as gamma radiation for "radiotrophic" growth. [63] It has been proposed that this process might bear some similarity to photosynthesis in plants, [63] but detailed biochemical data supporting the existence of this hypothetical pathway are presently lacking.
Morphology
Microscopic structures

Though fungi are part of the opisthokont clade, all phyla except for the chytrids have lost their posterior flagella.[64] Fungi are unusual among the eukaryotes in having a cell wall that, besides glucans (e.g., β-1,3-glucan) and other typical components, contains the biopolymer chitin.[65]
Many fungi grow as thread-like filamentous microscopic structures called hyphae, and an assemblage of intertwined and interconnected hyphae is called a mycelium. [6] Hyphae can be septate, i.e., divided into hyphal compartments separated by a septum, each compartment containing one or more nuclei or can be coenocytic, i.e., lacking hyphal compartmentalization. However, septa have pores, such as the doliporus in the basidiomycetes that allow cytoplasm, organelles, and sometimes nuclei to pass through.[6] Coenocytic hyphae are essentially multinucleate supercells.[66] In some cases, fungi have developed specialized structures for nutrient uptake from living hosts; examples include haustoria in plant-parasitic fungi of nearly all divisions, and arbuscules of several mycorrhizal fungi,[67] which penetrate into the host cells for nutrient uptake by the fungus.
Macroscopic structures
Fungal mycelia can become visible macroscopically, for example, as concentric rings on various surfaces, such as damp walls, and on other substrates, such as spoilt food (see figure), and are commonly and generically called mould (American spelling, mold); fungal mycelia grown on solid agar media in laboratory petri dishes are usually referred to as colonies, with many species exhibiting characteristic macroscopic growth morphologies and colours, due to spores or pigmentation.
Specialized fungal structures important in sexual reproduction are the apothecia, perithecia, and cleistothecia in the ascomycetes, and the fruiting bodies of the basidiomycetes, and a few ascomycetes. These reproductive structures can sometimes grow very large, and are well known as mushrooms.
Morphological and physiological features for substrate penetration
Fungal hyphae are specifically adapted to growth on solid surfaces and within substrates, and can exert astoundingly large penetrative mechanical forces. The plant pathogen, Magnaporthe grisea, forms a structure called an appressorium specifically designed for penetration of plant tissues, and the pressure generated by the appressorium, which is directed against the plant epidermis can exceed 8 MPa (80 bars). [68] The generation of these mechanical pressures is the result of an interplay between physiological processes to increase intracellular turgor by production of osmolytes such as glycerol, and the morphology of the appressorium. [69]
Reproduction

Reproduction of fungi is complex, reflecting the heterogeneity in lifestyles and genetic make up within this group of organisms. [6] Many fungi reproduce either sexually or asexually, depending on conditions in the environment. These conditions trigger genetically determined developmental programs leading to the expression of specialized structures for sexual or asexual reproduction. These structures aid both reproduction and efficient dissemination of spores or spore-containing propagules.
Asexual reproduction
Asexual reproduction via vegetative spores or through mycelial fragmentation is common in many fungal species and allows more rapid dispersal than sexual reproduction. In the case of the "Fungi imperfecti" or Deuteromycota, which lack a sexual cycle, it is the only means of propagation. Asexual spores, upon germination, may found a population that is clonal to the population from which the spore originated, and thus colonize new environments.
Sexual reproduction
Sexual reproduction with meiosis exists in all fungal phyla, except the Deuteromycota. It differs in many aspects from sexual reproduction in animals or plants. Many differences also exist between fungal groups and have been used to discriminate fungal clades and species based on morphological differences in sexual structures and reproductive strategies. Experimental crosses between fungal isolates can also be used to identify species based on biological species concepts. The major fungal clades have initially been delineated based on the morphology of their sexual structures and spores; for example, the spore-containing structures, asci and basidia, can be used in the identification of ascomycetes and basidiomycetes, respectively. Many fungal species have elaborate vegetative incompatibility systems that allow mating only between individuals of opposite mating type, while others can mate and sexually reproduce with any other individual or itself. Species of the former mating system are called heterothallic, and of the latter homothallic. [70]
Most fungi have both a haploid and diploid stage in their life cycles. In all sexually reproducing fungi, compatible individuals combine by cell fusion of vegetative hyphae by anastomosis, required for the initiation of the sexual cycle. Ascomycetes and basidiomycetes go through a dikaryotic stage, in which the nuclei inherited from the two parents do not fuse immediately after cell fusion, but remain separate in the hyphal cells (see heterokaryosis).
In ascomycetes, dikaryotic hyphae of the hymenium form a characteristic hook at the hyphal septum. During cell division formation of the hook ensures proper distribution of the newly divided nuclei into the apical and basal hyphal compartments. An ascus (plural asci) is then formed, in which karyogamy (nuclear fusion) occurs. These asci are embedded in an ascocarp, or fruiting body, of the fungus. Karyogamy in the asci is followed immediately by meiosis and the production of ascospores. The ascospores are disseminated and germinate and may form a new haploid mycelium.[71]
Sexual reproduction in basidiomycetes is similar to that of the ascomycetes. Compatible haploid hyphae fuse to produce a dikaryotic mycelium. However, the dikaryotic phase is more extensive in the basidiomycetes, in many cases also present in the vegetatively growing mycelium. A specialized anatomical structure, called a clamp connection, is formed at each hyphal septum. As with the structurally similar hook in the ascomycetes, formation of the clamp connection in the basidiomycetes is required for controlled transfer of nuclei during cell division, to maintain the dikaryotic stage with two genetically different nuclei in each hyphal compartment. [71] A basidiocarp is formed in which club-like structures known as basidia generate haploid basidiospores after karyogamy and meiosis.[72] The most commonly known basidiocarps are mushrooms, but they may also take many other forms (see Morphology section).
In zygomycetes, haploid hyphae of two individuals fuse, forming a zygote, which develops into a zygospore. When the zygospore germinates, it quickly undergoes meiosis, generating new haploid hyphae, which in turn may form asexual sporangiospores. These sporangiospores are means of rapid dispersal of the fungus and germinate into new genetically identical haploid fungal colonies, able to mate and undergo another sexual cycle followed by the generation of new zygospores, thus completing the lifecycle.
Spore dispersal
Both asexual and sexual spores or sporangiospores of many fungal species are actively dispersed by forcible ejection from their reproductive structures. This ejection ensures exit of the spores from the reproductive structures as well as travelling through the air over long distances. Many fungi thereby possess specialized mechanical and physiological mechanisms as well as spore-surface structures, such as hydrophobins, for spore ejection. These mechanisms include, for example, forcible discharge of ascospores enabled by the structure of the ascus and accumulation of osmolytes in the fluids of the ascus that lead to explosive discharge of the ascospores into the air. [73] The forcible discharge of single spores termed ballistospores involves formation of a small drop of water (Buller's drop), which upon contact with the spore leads to its projectile release with an initial acceleration of more than 10,000 g. [74] Other fungi rely on alternative mechanisms for spore release, such as external mechanical forces, exemplified by puffballs. Attracting insects, such as flies, to fruiting structures, by virtue of their having lively colours and a putrid odour, for dispersal of fungal spores is yet another strategy, most prominently used by the stinkhorns.
Other sexual processes
Besides regular sexual reproduction with meiosis, some fungal species may exchange genetic material via parasexual processes, initiated by anastomosis between hyphae and plasmogamy of fungal cells. The frequency and relative importance of parasexual events is unclear and may be lower than other sexual processes. However, it is known to play a role in intraspecific hybridization [75] and is also likely required for hybridization between fungal species, which has been associated with major events in fungal evolution. [76]
Phylogeny and classification

For a long time taxonomists considered fungi to be members of the Plant Kingdom. This early classification was based mainly on similarities in lifestyle: both fungi and plant are mainly sessile, have similarities in general morphology and growth habitat (like plants, fungi often grow in soil, in the case of mushrooms forming conspicuous fruiting bodies, which sometimes bear resemblance to plants such as mosses). Moreover, both groups possess a cell wall, which is absent in the Animal Kingdom. However, the fungi are now considered a separate kingdom, distinct from both plants and animals, from which they appear to have diverged approximately one billion years ago.[77] Many studies have identified several distinct morphological, biochemical, and genetic features in the Fungi, clearly delineating this group from the other kingdoms. For these reasons, the fungi are placed in their own kingdom.
Physiological and morphological traits
Similar to animals and unlike most plants, fungi lack the capacity to synthesize organic carbon by chlorophyll-based photosynthesis; whereas plants store the reduced carbon as starch, fungi, like animals and some bacteria, use glycogen [78] for storage of carbohydrates. A major component of the cell wall in many fungal species is the nitrogen-containing carbohydrate, chitin,[79] also present in some animals, such as the insects and crustaceans, while the plant cell wall consists chiefly of the carbohydrate cellulose. The defining and unique characteristics of fungal cells include growth as hyphae, which are microscopic filaments of between 2-10 microns in diameter and up to several centimetres in length, and which combined form the fungal mycelium. Some fungi, such as yeasts, grow as single ovoid cells, similar to unicellular algae and the protists.
Unlike many plants, most fungi lack an efficient vascular system, such as xylem or phloem for long-distance transport of water and nutrients; as an example for convergent evolution, some fungi, such as Armillaria, form rhizomorphs or mycelial cords,[80] resembling and functionally related to, but morphologically distinct from, plant roots.
Some characteristics shared between plants and fungi include the presence of vacuoles in the cell,[81] and a similar pathway in the biosynthesis of terpenes using mevalonic acid and pyrophosphate as biochemical precursors; plants however use an additional terpene biosynthesis pathway in the chloroplasts that is apparently absent in fungi.[82] Ancestral traits shared among members of the fungi include chitinous cell walls and heterotrophy by absorption.[71] A further characteristic of the fungi that is absent from other eukaryotes, and shared only with some bacteria, is the biosynthesis of the amino acid, L-lysine, via the α-aminoadipate pathway. [83]
Similar to plants, fungi produce a plethora of secondary metabolites functioning as defensive compounds or for niche adaptation; however, biochemical pathways for the synthesis of similar or even identical compounds often differ markedly between fungi and plants. [84][85]
Evolutionary history
The first organisms having features typical of fungi date to 1200, the Proterozoic.[86] However, fungal fossils do not become common and uncontroversial until the early Devonian, when they are abundant in the Rhynie chert.[87]
Even though traditionally included in many botany curricula and textbooks, fungi are now thought to be more closely related to animals than to plants and are placed with the animals in the monophyletic group of opisthokonts.[71] For much of the Paleozoic Era, the fungi appear to have been aquatic, and consisted of organisms similar to the extant Chytrids in having flagellum-bearing spores.[88] The early fossil record of the fungi is fragmentary, to say the least. The fungi probably colonized the land during the Cambrian, long before land plants.[87] For some time after the Permian-Triassic extinction event, a fungal spike, originally thought to be an extraordinary abundance of fungal spores in sediments formed shortly after this event, suggested that they were the dominant life form during this period—nearly 100% of the fossil record available from this period.[89] However, the relative proportion of fungal spores relative to spores formed by algal species is difficult to assess, [90] the spike did not appear world-wide, [91][92] and in many places it did not fall on the Permian-Triassic boundary.[93]
Analyses using molecular phylogenetics support a monophyletic origin of the Fungi.[8] The taxonomy of the Fungi is in a state of constant flux, especially due to recent research based on DNA comparisons. These current phylogenetic analyses often overturn classifications based on older and sometimes less discriminative methods based on morphological features and biological species concepts obtained from experimental matings.[94][95]
There is no unique generally accepted system at the higher taxonomic levels and there are constant name changes at every level, from species upwards. However, efforts among fungal researchers are now underway to establish and encourage usage of a unified and more consistent nomenclature.[8] Fungal species can also have multiple scientific names depending on its life cycle and mode (sexual or asexual) of reproduction. Web sites such as Index Fungorum and ITIS define preferred up-to-date names (with cross-references to older synonyms), but do not always agree with each other.
Cladogram
The taxonomic groups of fungi
The major divisions (phyla) of fungi have been classified based mainly on their sexual reproductive structures. Currently, seven fungal divisions are proposed:[8]

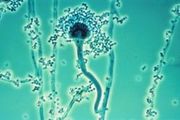
- The Chytridiomycota are commonly known as chytrids. These fungi are ubiquitous with a worldwide distribution; chytrids produce zoospores that are capable of active movement through aqueous phases with a single flagellum. Consequently, some taxonomists had earlier classified them as protists on the basis of the flagellum. Molecular phylogenies, inferred from the rRNA-operon sequences representing the 18S, 28S, and 5.8S ribosomal subunits, suggest that the Chytrids are a basal fungal group divergent from the other fungal divisions, consisting of four major clades with some evidence for paraphyly or possibly polyphyly. [88]
- The Blastocladiomycota were previously considered a taxonomic clade within the Chytridiomycota. Recent molecular data and ultrastructural characteristics, however, place the Blastocladiomycota as a sister clade to the Zygomycota, Glomeromycota, and Dikarya (Ascomycota and Basiomycota). The blastocladiomycetes are fungi that are saprotrophs and parasites of all eukaryotic groups and undergo sporic meiosis unlike their close relatives, the chytrids, which mostly exhibit zygotic meiosis. [88]
- The Neocallimastigomycota were earlier placed in the phylum Chytridomycota. Members of this small phylum are anaerobic organisms, living in the digestive system of larger herbivorous mammals and possibly in other terrestrial and aquatic environments. They lack mitochondria but contain hydrogenosomes of mitochondrial origin. As the related chrytrids, neocallimastigomycetes form zoospores that are posteriorly uniflagellate or polyflagellate.[8]
- The Zygomycota contain the taxa, Zygomycetes and Trichomycetes, and reproduce sexually with meiospores called zygospores and asexually with sporangiospores. Black bread mold (Rhizopus stolonifer) is a common species that belongs to this group; another is Pilobolus, which is capable of ejecting spores several meters through the air. Medically relevant genera include Mucor, Rhizomucor, and Rhizopus. Molecular phylogenetic investigation has shown the Zygomycota to be a polyphyletic phylum with evidence of paraphyly within this taxonomic group. [96]
- Members of the Glomeromycota are fungi forming arbuscular mycorrhizae with higher plants. Only one species has been observed forming zygospores; all other species solely reproduce asexually. The symbiotic association between the Glomeromycota and plants is ancient, with evidence dating to 400 million years ago.[45]

- The Ascomycota, commonly known as sac fungi or ascomycetes, constitute the largest taxonomic group within the Eumycota. These fungi form meiotic spores called ascospores, which are enclosed in a special sac-like structure called an ascus. This division includes morels, a few mushrooms and truffles, single-celled yeasts (e.g., of the genera Saccharomyces, Kluyveromyces, Pichia, and Candida), and many filamentous fungi living as saprotrophs, parasites, and mutualistic symbionts. Prominent and important genera of filamentous ascomycetes include Aspergillus, Penicillium, Fusarium, and Claviceps. Many ascomycetes species have only been observed undergoing asexual reproduction (called anamorphic species), but molecular data has often been able to identify their closest teleomorphs in the Ascomycota. Because the products of meiosis are retained within the sac-like ascus, several ascomyctes have been used for elucidating principles of genetics and heredity (e.g. Neurospora crassa).
- Members of the Basidiomycota, commonly known as the club fungi or basidiomycetes, produce meiospores called basidiospores on club-like stalks called basidia. Most common mushrooms belong to this group, as well as rust (fungus) and smut fungi, which are major pathogens of grains. Other important Basidiomyces include the maize pathogen,Ustilago maydis, human commensal species of the genus Malassezia, and the opportunistic human pathogen, Cryptococcus neoformans.
Phylogenetic relationships with other fungus-like organisms
Because of some similarities in morphology and lifestyle, the slime molds (myxomycetes) and water molds (oomycetes) were formerly classified in the kingdom Fungi. Unlike true fungi, however, the cell walls of these organisms contain cellulose and lack chitin. Slime molds are unikonts like fungi, but are grouped in the Amoebozoa. Water molds are diploid bikonts, grouped in the Chromalveolate kingdom. Neither water molds nor slime molds are closely related to the true fungi, and, therefore, taxonomists no longer group them in the kingdom Fungi. Nonetheless, studies of the oomycetes and myxomycetes are still often included in mycology textbooks and primary research literature.
It has been suggested that the nucleariids, currently grouped in the Choanozoa, may be a sister group to the oomycete clade, and as such could be included in an expanded fungal kingdom.[97]
See also
- Bioaerosol
- Carnivorous fungus
- Entomopathogenic fungi
- Fusicoccin
- List of fungal orders
- MycoBank
- Plant pathology
- Wood-decay fungus
- Pathogenic fungi
Notes and references
- ↑ "Taxonomic proposals for the classification of marine yeasts and other yeast-like fungi including the smuts". Bot. Mar. 23: 371. 1980.
- ↑ These are the pronunciations listed first in most dictionaries. See, for example, the Merriam-Webster Online entry Alternative pronunciations for fungi include /ˈfʌŋgaɪ/, /ˈfʌndʒi/, and /ˈfʌŋgi/. Funguses (/ˈfʌŋgəsəz/) is an alternative plural form.
- ↑ Simpson, D.P. (1979). Cassell's Latin Dictionary (5 ed.). London: Cassell Ltd. p. 883. ISBN 0-304-52257-0.
- ↑ Hawksworth DL (2006). "The fungal dimension of biodiversity: magnitude, significance, and conservation". Mycol. Res. 95: 641–655.
- ↑ Mueller GM, Schmit JP (2006). "Fungal biodiversity: what do we know? What can we predict?". Biodivers Conserv. 16: 1–5.
- ↑ 6.0 6.1 6.2 6.3 Alexopoulos CJ, Mims CW, Blackwell M (1996). Introductory Mycology. John Wiley and Sons. ISBN 0471522295.
- ↑ Meredith Blackwell (2005-02-14). "Eumycota: mushrooms, sac fungi, yeast, molds, rusts, smuts, etc". Retrieved 2007-04-06. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ 8.0 8.1 8.2 8.3 8.4 Hibbett, D.S.; et al. (2007). "A higher level phylogenetic classification of the Fungi". Mycol. Res. 111 (5): 509–547. doi:doi:10.1016/j.mycres.2007.03.004 Check
|doi=value (help). - ↑ Strains of wine yeast
- ↑ Demain AL. (1991). "Production of beta-lactam antibiotics and its regulation". Proc Natl Sci Counc Repub China B. 15: 251–265. PMID 1815263.
- ↑ Kulp, Karel (2000). Handbook of Cereal Science and Technology. CRC Press. ISBN 0824782941.
- ↑ Piskur J, Rozpedowska E, Polakova S, Merico A, Compagno C. (2006). "How did Saccharomyces evolve to become a good brewer?". Trends Genet. 22: 183–186. PMID 16499989.
- ↑ Kitamoto N, Yoshino S, Ohmiya K, Tsukagoshi N. (1999). "Sequence analysis, overexpression, and antisense inhibition of a beta-xylosidase gene, xylA, from Aspergillus oryzae KBN616". Appl. Env. Microbiol. 65: 20–24. PMID 9872754.
- ↑ "Trichoderma spp., including T. harzianum, T. viride, T. koningii, T. hamatum and other spp. Deuteromycetes, Moniliales (asexual classification system)". Biological Control: A Guide to Natural Enemies in North America. Retrieved 2007-07-10.
- ↑ van Egmond HP, Schothorst RC, Jonker MA (2007). "Regulations relating to mycotoxins in food: perspectives in a global and European context". Anal Bioanal Chem. 389: 147–157. PMID 17508207.
- ↑ Keller NP, Turner G, Bennett JW (2005). "Fungal secondary metabolism - from biochemistry to genomics". Nat Rev Microbiol. 3: 937–497. PMID 16322742.
- ↑ Demain AL, Fang A (2000). "The natural functions of secondary metabolites". Adv Biochem Eng Biotechnol. 69: 1–39. PMID 11036689.
- ↑ Rohlfs M, Albert M, Keller NP, Kempken F (2007). "Secondary chemicals protect mould from fungivory". Biol Lett. 3: 523–525. PMID 17686752.
- ↑ Questions & Answers - Mold on Cheese whatscookingamerica.net. Retrieved 2007-04-06.
- ↑ Erdogan A, Gurses M, Sert S. (2004). "Isolation of moulds capable of producing mycotoxins from blue mouldy Tulum cheeses produced in Turkey". Int J Food Microbiol. 85: 83–85. PMID 12810273.
- ↑ On the Trail of the Death Cap Mushroom Richard Harris, www.npr.org, 2007-02-08. Retrieved 2007-04-06.
- ↑ Leathem AM, Dorran TJ (2007). "Poisoning due to raw Gyromitra esculenta (false morels) west of the Rockies". CJEM. 9: 127–130. PMID 17391587.
- ↑ Karlson-Stiber C, Persson H (2003). "Cytotoxic fungi--an overview". Toxicon. 42: 339–349. PMID 14505933.
- ↑ Mythology and Folklore of Fly Agaric Paul Kendall, Trees for Life. Retrieved 2007-04-06.
- ↑ López-Gómez J, Molina-Meyer M (2006). "The competitive exclusion principle versus biodiversity through competitive segregation and further adaptation to spatial heterogeneities". Theor Popul Biol. 69: 94–109. PMID 16223517.
- ↑ Setting the Stage To Screen Biocontrol Fungi Hank Becker, July 1998. Retrieved 2007-04-06.
- ↑ WHEY-BASED FUNGAL MICROFACTORY TECHNOLOGY FOR ENHANCED BIOLOGICAL PEST MANAGEMENT USING FUNGI Todd. S. Keiller, Technology Transfer, University of Vermont. Retrieved 2007-04-06.
- ↑ Deshpande MV. (1999). "Mycopesticide production by fermentation: potential and challenges". Crit Rev Microbiol. 25: 229–243. PMID 10524330.
- ↑ Thomas MB, Read AF. (2007). "Can fungal biopesticides control malaria?". Nat Rev Microbiol. 5: 377–383. doi:10.1038/nrmicro1638. PMID 17426726.
- ↑ Bush LP, Wilkinson HH, Schardl CL. (1997). "Bioprotective Alkaloids of Grass-Fungal Endophyte Symbioses". Plant Physiol. 114: 1–7. PMID 12223685.
- ↑ Bouton JH, Latch GCM, Hill NS, Hoveland CS, McCannc MA, Watson RH, Parish JA, Hawkins LL, Thompson FN (2002). "Use of nonergot alkaloid-producing endophytes for alleviating tall fescue toxicosis in sheep". Agron. J. 94: 567–574. http://agron.scijournals.org/cgi/content/full/94/3/567.
- ↑ Douglas, M.S. (1995). Bioremediation of Contaminated Soils Using the White Rot Fungus Phanerochaete Chrysosporium. DTIC.
- ↑ BBC. (2008). Fungi to fight 'toxic war zones'
- ↑ Lindahl BD, Ihrmark K, Boberg J, Trumbore SE, Högberg P, Stenlid J, Finlay RD (2007). "Spatial separation of litter decomposition and mycorrhizal nitrogen uptake in a boreal forest". New Phytol. 173: 611–620. PMID 17244056.
- ↑ Barea JM, Pozo MJ, Azcón R, Azcón-Aguilar C (2005). "Microbial co-operation in the rhizosphere". J. Exp. Bot. 56: 1761–1778. PMID 15911555.
- ↑ Aanen DK. (2006). "As you reap, so shall you sow: coupling of harvesting and inoculating stabilizes the mutualism between termites and fungi". Biol Lett. 2: 209–212. PMID 17148364.
- ↑ Nikoh N, Fukatsu T. (2000). "Interkingdom host jumping underground: phylogenetic analysis of entomoparasitic fungi of the genus Cordyceps". Mol Biol Evol. 17: 2629–2638. PMID 10742053.
- ↑ Perotto S, Bonfante P. (1997). "Bacterial associations with mycorrhizal fungi: close and distant friends in the rhizosphere". Trends Microbiol. 5: 496–501. PMID 9447662.
- ↑ Arnold AE, Mejía LC, Kyllo D, Rojas EI, Maynard Z, Robbins N, Herre EA. (2003). "Fungal endophytes limit pathogen damage in a tropical tree". Proc. Natl. Acad. Sci. USA. 100: 15649–15654. PMID 14671327.
- ↑ 40.0 40.1 Paszkowski U. (2006). "Mutualism and parasitism: the yin and yang of plant symbioses". Curr Opin Plant Biol. 9: 364–370. PMID 16713732.
- ↑ 41.0 41.1 Hube B. (2004). "From commensal to pathogen: stage- and tissue-specific gene expression of Candida albicans". Curr Opin Microbiol. 7: 336–341. PMID 15288621.
- ↑ Volk, Tom. "Tom Volk's Fungi FAQ". Retrieved 2006-09-21.
- ↑ Wong, George. "Symbiosis: Mycorrhizae and Lichens". Retrieved 2006-09-21.
- ↑ Knowledge of nitrogen transfer between plants and beneficial fungi expands southwestfarmpress.com. 2005-06-10 Retrieved 2007-04-06.
- ↑ 45.0 45.1 Remy W, Taylor TN, Hass H, Kerp H (1994). "4-hundred million year old vesicular-arbuscular mycorrhizae". Proc. Natl. Acad. Sci. 91: 11841–11843. PMID 11607500.
- ↑ Selosse MA, Richard F, He X, Simard SW (2006). "Mycorrhizal networks: des liaisons dangereuses?". Trends Ecol Evol. 21: 621–628. PMID 16843567.
- ↑ Brodo, Irwin M. (2001). Lichens of North America. Yale University Press. ISBN 0300082495. Unknown parameter
|coauthors=ignored (help) - ↑ Fungi and Insect Symbiosis www.botany.hawaii.edu. Retrieved 2007-04-06.
- ↑ Pascal Jouquet, Virginie Tavernier, Luc Abbadie and Michel Lepage. Nests of subterranean fungus-growing termites (Isoptera, Macrotermitinae) as nutrient patches for grasses in savannah ecosystems. African Journal of Ecology. 2005. Vol 43, 191–196
- ↑ Talbot NJ (2003). "On the trail of a cereal killer: Exploring the biology of Magnaporthe grisea". Annu Rev Microbiol. 57: 177–202. PMID 14527276.
- ↑ Paoletti M, Buck KW, Brasier CM. (2006). "Selective acquisition of novel mating type and vegetative incompatibility genes via interspecies gene transfer in the globally invading eukaryote Ophiostoma novo-ulmi". Mol Ecol. 15: 249–262. PMID 16367844.
- ↑ Gryzenhout M, Wingfield BD, Wingfield MJ. (2006). "New taxonomic concepts for the important forest pathogen Cryphonectria parasitica and related fungi". FEMS Microbiol Lett. 258: 161–172. PMID 16640568.
- ↑ Nielsen K, Heitman J. (2007). "Sex and virulence of human pathogenic fungi". Adv Genet. 57: 143–173. PMID 17352904.
- ↑ Brakhage AA (2005). "Systemic fungal infections caused by Aspergillus species: epidemiology, infection process and virulence determinants". Curr. Drug Targets. 6: 875–886. doi:10.2174/138945005774912717. PMID 16375671.
- ↑ Kauffman CA. (2007). "Histoplasmosis: a clinical and laboratory update". Clin Microbiol Rev. 20: 115–132. PMID 17223625.
- ↑ Cushion MT, Smulian AG, Slaven BE, Sesterhenn T, Arnold J, Staben C, Porollo A, Adamczak R, Meller J. (2007). "Transcriptome of Pneumocystis carinii during Fulminate Infection: Carbohydrate Metabolism and the Concept of a Compatible Parasite". PLoS ONE. 2: e423. doi:10.1371/journal.pone.0000423. PMID 17487271.
- ↑ ILLUSTRATIONS for Predatory Fungi, wood Decay and the Carbon Cycle www.uoguelph.ca. Retrieved 2007-04-06.
- ↑ Pereira JL, Noronha EF, Miller RN, Franco OL. (2007). "Novel insights in the use of hydrolytic enzymes secreted by fungi with biotechnological potential". Lett Appl Microbiol. 44: 573–581. PMID 17576216.
- ↑ Schaller M, Borelli C, Korting HC, Hube B. (2007). "Hydrolytic enzymes as virulence factors of Candida albicans". Mycoses. 48: 365–377. PMID 16262871.
- ↑ Farrar JF (1985). "Carbohydrate metabolism in biotrophic plant pathogens". Microbiol Sci. 2: 314–317. PMID 3939987.
- ↑ Marzluf GA (1981). "Regulation of nitrogen metabolism and gene expression in fungi". Microbiol Rev. 45: 437–461. PMID 6117784.
- ↑ Heynes MJ (1994). "Regulatory circuits of the amdS gene of Aspergillus nidulans". Antonie Van Leeuwenhoek. 65: 179–782. PMID 7847883.
- ↑ 63.0 63.1 Dadachova E, Bryan RA, Huang X, Moadel T, Schweitzer AD, Aisen P, Nosanchuk JD, Casadevall A. (2007). "Ionizing radiation changes the electronic properties of
melanin and enhances the growth of melanized fungi". PLoS ONE. 2: e457. PMID 17520016. line feed character in
|title=at position 56 (help) - ↑ The Protistan Origins of Animals and Fungi Emma T. Steenkamp, Jane Wright and Sandra L. Baldauf. Molecular Biology and Evolution 2006 23(1):93-106; doi:10.1093/molbev/msj011. Retrieved 2007-04-06.
- ↑ Stevens DA, Ichinomiya M, Koshi Y, Horiuchi H. (2006). "Escape of Candida from caspofungin inhibition at concentrations above the MIC (paradoxical effect) accomplished by increased cell wall chitin; evidence for beta-1,6-glucan synthesis inhibition by caspofungin". Antimicrob Agents Chemother. 50: 3160-3161. PMID 16940118.
- ↑ Chang, Shu-ting (2004). Mushrooms: Cultivation, Nutritional Value, Medicinal Effect and Environmental Impact. CRC Press. ISBN 0849310431. Unknown parameter
|coauthors=ignored (help) - ↑ “Fungal Biology” at The University of Sydney Retrieved on 26 June 2007
- ↑ Howard RJ, Ferrari MA, Roach DH, Money NP (1991). "Penetration of hard substrates by a fungus employing enormous turgor pressures". Proc Natl Acad Sci U S A. 88: 11281–11284. PMID 1837147.
- ↑ Wang ZY, Jenkinson JM, Holcombe LJ, Soanes DM, Veneault-Fourrey C, Bhambra GK, Talbot NJ (2005). "The molecular biology of appressorium turgor generation by the rice blast fungus Magnaporthe grisea". Biochem Soc Trans. 33: 384–388. PMID 15787612.
- ↑ Metzenberg RL, Glass NL. (1990). "Mating type and mating strategies in Neurospora". Bioessays. 12: 53–59. PMID 2140508.
- ↑ 71.0 71.1 71.2 71.3 P. Sitte, H. Ziegler, F. Ehrendorfer (1991). Strasburger Lehrbuch der Botanik (Textbook of Botany) (33 ed ed.). Urban & Fischer. ISBN 3437204475.
- ↑ Reproduction of fungi MicrobiologyBytes, 2007-01-18. Retrieved 2007-04-06.
- ↑ Trail F. (2007). "Fungal cannons: explosive spore discharge in the Ascomycota". FEMS Microbiol Lett. 276: 12–18. PMID 17784861.
- ↑ Pringle A, Patek SN, Fischer M, Stolze J, Money NP. (2005). "The captured launch of a ballistospore". Mycologia. 97: 866–871. PMID 16457355.
- ↑ Furlaneto MC, Pizzirani-Kleiner AA. (1992). "Intraspecific hybridisation of Trichoderma pseudokoningii by anastomosis and by protoplast fusion". FEMS Microbiol Lett. 69: 191–195. PMID 1537549.
- ↑ Schardl CL, Craven KD. (2003). "Interspecific hybridization in plant-associated fungi and oomycetes: a review". Mol. Ecol. 12: 2861–2873. PMID 14629368.
- ↑ Bruns T. (2006). "Evolutionary biology: a kingdom revised". Nature. 443: 758–761. doi:10.1038/443758a%3Ca. PMID 17051197. Unknown parameter
|doilabel=ignored (help) - ↑ Lomako J, Lomako WM, Whelan WJ. (2004). "Glycogenin: the primer for mammalian and yeast glycogen synthesis". Biochim Biophys Acta. 1673: 45–55. PMID 15238248.
- ↑ Bowman SM, Free SJ. (2006). "The structure and synthesis of the fungal cell wall". Bioessays. 28: 799–808. PMID 16927300.
- ↑ Mihail JD, Bruhn JN. (2005). "Foraging behaviour of Armillaria rhizomorph systems". Mycol. Res. 109: 1195–1207. PMID 16279413.
- ↑ Shoji JY, Arioka M, Kitamoto K (2006). "Possible involvement of pleiomorphic vacuolar networks in nutrient recycling in filamentous fungi". Autophagy. 2: 226–227. PMID 16874107.
- ↑ Wu S, Schalk M, Clark A, Miles RB, Coates R, Chappell J. (2007). "Redirection of cytosolic or plastidic isoprenoid precursors elevates terpene production in plants". Nat Biotechnol. 24: 1441–7. PMID 17057703.
- ↑ Xu H, Andi B, Qian J, West AH, Cook PF (2006). "The alpha-aminoadipate pathway for lysine biosynthesis in fungi". Cell Biochem Biophys. 46: 43–64. PMID 16943623.
- ↑ Tudzynski B. (2005). "Gibberellin biosynthesis in fungi: genes, enzymes, evolution, and impact on biotechnology". Appl Microbiol Biotechnol. 66: 597–611. PMID 15578178.
- ↑ Siewers V, Smedsgaard J, Tudzynski P. (2004). "The P450 monooxygenase BcABA1 is essential for abscisic acid biosynthesis in Botrytis cinerea". Appl Environ. Microbiol. 70: 3868–3876. doi:10.1128/AEM.70.7.3868-3876.2004. PMID 15240257.
- ↑ Butterfield, N.J. (2005). "Probable Proterozoic fungi". Paleobiology. 31 (1): 165–182. doi:... Check
|doi=value (help). Unknown parameter|doilabel=ignored (help);|access-date=requires|url=(help) - ↑ 87.0 87.1 Brundrett, M.C. (2002). "Coevolution of roots and mycorrhizas of land plants". New Phytologist. 154 (2): 275–304. doi:10.1046/j.1469-8137.2002.00397.x.
- ↑ 88.0 88.1 88.2 James TY; et al. (2006). "Reconstructing the early evolution of Fungi using a six-gene phylogeny". Nature. 443: 818–822. doi:10.1038/nature05110. PMID 17051209.
- ↑ Eshet, Y. et al. (1995) Fungal event and palynological record of ecological crisis and recovery across the Permian-Triassic boundary. Geology, 23, 967-970.
- ↑ Foster, C.B. (2002). "A Revision Of Reduviasporonites Wilson 1962: Description, Illustration, Comparison And Biological Affinities". Palynology. 26 (1): 35–58. doi:10.2113/0260035. Unknown parameter
|coauthors=ignored (help) - ↑ López-Gómez, J. and Taylor, E.L. (2005). "Permian-Triassic Transition in Spain: A multidisciplinary approach". Palaeogeography, Palaeoclimatology, Palaeoecology. 229 (1–2): 1–2. doi:10.1016/j.palaeo.2005.06.028.
- ↑ Looy, C.V. (2005). "Life in the end-Permian dead zone". Proceedings of the National Academy of Sciences. 162 (4): 653–659. doi:10.1073/pnas.131218098.
See image 2
Unknown parameter|coauthors=ignored (help) - ↑ Ward PD, Botha J, Buick R, De Kock MO, Erwin DH, Garrison GH, Kirschvink JL & Smith R (2005). "Abrupt and Gradual Extinction Among Late Permian Land Vertebrates in the Karoo Basin, South Africa". Science. 307 (5710): 709–714. doi:10.1126/science.1107068.
- ↑ See Palaeos: Fungi for an introduction to fungal taxonomy, including recent controversies.
- ↑ “A Higher-Level Phylogenetic Classification of the Fungi” by David S. Hibbett, (.pdf file) Retrieved on 8 March 2007
- ↑ White MM, James TY, O'Donnell K, Cafaro MJ, Tanabe Y, Sugiyama J. (2006). "Phylogeny of the Zygomycota based on nuclear ribosomal sequence data". Mycologia. 98: 872–884. PMID 17486964.
- ↑ Esser, Karl (1994). The Mycota: A Comprehensive Treatise on Fungi as Experimental Systems for Basic and Applied Research. Springer. ISBN 3540580085. Unknown parameter
|coauthors=ignored (help)
Further reading
- Alexopoulos, C.J., Charles W. Mims, M. Blackwell et al., Introductory Mycology, 4th ed. (John Wiley and Sons, Hoboken NJ, 2004) ISBN 0-471-52229-5
- Arora, David. (1986). "Mushrooms Demystified: A Comprehensive Guide to the Fleshy Fungi". 2nd ed. Ten Speed Press. ISBN 0898151694
- Deacon JW. (2005). "Fungal Biology" (4th ed). Malden, MA: Blackwell Publishers. ISBN 1-4051-3066-0.
- Kaminstein D. (2002). Mushroom poisoning.
External links
- The WWW Virtual Library: Mycology
- MykoWeb
- Illinois Mycological Association Mycological Glossary
- Tree of Life web project: Fungi
- Fungal Biology, University of Sydney, School of Biological Sciences, June, 2004. – Online textbook
- The Fifth Kingdom – Online textbook
- CABI Bioscience Databases - Includes Index Fungorum genus and species names and top-down hierarchy
- Comparative Analysis of Fungal Genomes (at DOE's IMG system)
- Fungi Bioluminescence Laboratory - Chemistry Institute, University of São Paulo, Brazil
Template:Link FA ar:فطر ast:Fungi bn:ছত্রাক zh-min-nan:Ko͘ bg:Гъби ca:Fong cv:Кăмпа cs:Houby cy:Ffwng da:Svampe de:Pilze et:Seened el:Μύκητας eo:Fungoj ga:Fungas ko:균계 hi:कवक hsb:Hriby hr:Gljive id:Fungi is:Sveppur it:Funghi he:פטריות la:Fungi lv:Sēnes lb:Pilzeräich lt:Grybai hu:Gombák mk:Габа ms:Kulat nah:Nanacatl nl:Schimmels no:Sopper nn:Sopp oc:Mycota nds:Poggenstöhl qu:K'allampa scn:Funci simple:Fungus sk:Huby sl:Glive sr:Гљиве fi:Sienet sv:Svampar ta:பூஞ்சைகள் th:เห็ดรา uk:Гриб wa:Tchampion yi:פאנגוס bat-smg:Kremblē
- Pages with reference errors
- CS1 maint: Multiple names: authors list
- Pages with citations using unsupported parameters
- CS1 errors: dates
- CS1 errors: DOI
- CS1 maint: Explicit use of et al.
- CS1 errors: invisible characters
- CS1 maint: Extra text
- Pages using citations with accessdate and no URL
- CS1 maint: Uses authors parameter
- Fungi