Fosaprepitant: Difference between revisions
Kiran Singh (talk | contribs) No edit summary |
Kiran Singh (talk | contribs) No edit summary |
||
| Line 174: | Line 174: | ||
* [[Angioedema]] and [[urticaria]] were reported as serious adverse reactions in a patient receiving aprepitant in a non-CINV/non-PONV study. | * [[Angioedema]] and [[urticaria]] were reported as serious adverse reactions in a patient receiving aprepitant in a non-CINV/non-PONV study. | ||
|postmarketing=* The following adverse reactions have been identified during post-approval use of fosaprepitant and aprepitant. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. | |postmarketing=* The following adverse reactions have been identified during post-approval use of fosaprepitant and aprepitant. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. | ||
| Line 260: | Line 259: | ||
* Coadministration of once daily doses of aprepitant, as a tablet formulation comparable to 85 mg or 170 mg of the capsule formulation, with paroxetine 20 mg once daily, resulted in a decrease in AUC by approximately 25% and Cmax by approximately 20% of both aprepitant and paroxetine. | * Coadministration of once daily doses of aprepitant, as a tablet formulation comparable to 85 mg or 170 mg of the capsule formulation, with paroxetine 20 mg once daily, resulted in a decrease in AUC by approximately 25% and Cmax by approximately 20% of both aprepitant and paroxetine. | ||
|useInPregnancyFDA= | |useInPregnancyFDA='''Teratogenic effects''' | ||
'''Pregnancy Category B''': In the reproduction studies conducted with fosaprepitant and aprepitant, the highest systemic exposures to aprepitant were obtained following oral administration of aprepitant. Reproduction studies performed in rats at oral doses of aprepitant up to 1000 mg/kg twice daily (plasma AUC0-24hr of 31.3 mcg•hr/mL, about 1.6 times the human exposure at the recommended dose) and in rabbits at oral doses up to 25 mg/kg/day (plasma AUC0-24hr of 26.9 mcg•hr/mL, about 1.4 times the human exposure at the recommended dose) revealed no evidence of impaired fertility or harm to the fetus due to aprepitant. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. | |||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | ||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | ||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | ||
|useInNursing= | |useInNursing=* Aprepitant is excreted in the milk of rats. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for possible serious adverse reactions in nursing infants from aprepitant and because of the potential for tumorigenicity shown for aprepitant in rodent carcinogenicity studies, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. | ||
|useInPed= | |useInPed=* Safety and effectiveness of EMEND for Injection in pediatric patients have not been established. | ||
|useInGeri= | |useInGeri=* In 2 well-controlled chemotherapy-induced nausea and vomiting clinical studies, of the total number of patients (N=544) treated with oral aprepitant, 31% were 65 and over, while 5% were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects. Greater sensitivity of some older individuals cannot be ruled out. Dosage adjustment in the elderly is not necessary | ||
|useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | ||
|useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | ||
|useInRenalImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | |useInRenalImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | ||
|useInHepaticImpair=There | |useInHepaticImpair=* There are no clinical or pharmacokinetic data in patients with severe hepatic impairment (Child-Pugh score >9). Therefore, caution should be exercised when fosaprepitant or aprepitant is administered in these patients | ||
|useInReproPotential=There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |useInReproPotential=There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | ||
|useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | ||
| Line 287: | Line 288: | ||
<!--Overdosage--> | <!--Overdosage--> | ||
|overdose= | |overdose=* There is no specific information on the treatment of overdosage with fosaprepitant or aprepitant. | ||
* | * In the event of overdose, fosaprepitant and/or oral aprepitant should be discontinued and general supportive treatment and monitoring should be provided. Because of the antiemetic activity of aprepitant, drug-induced emesis may not be effective. | ||
* Aprepitant cannot be removed by hemodialysis. | |||
* | * Thirteen patients in the randomized controlled trial of EMEND for Injection received both fosaprepitant 150 mg and at least one dose of oral aprepitant, 125 mg or 80 mg. Three patients reported adverse reactions that were similar to those experienced by the total study population. | ||
|drugBox=<!--Mechanism of Action--> | |drugBox=<!--Mechanism of Action--> | ||
|mechAction=* | |mechAction=* | ||
<!--Structure--> | <!--Structure--> | ||
|structure= | |structure=EMEND (fosaprepitant dimeglumine) for Injection is a sterile, lyophilized prodrug of aprepitant, a substance P/neurokinin-1 (NK1) receptor antagonist, and is chemically described as 1-Deoxy-1-(methylamino)-D-glucitol[3-[[(2R,3S)-2-[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3-(4-fluorophenyl)-4-morpholinyl]methyl]-2,5-dihydro-5-oxo-1H-1,2,4-triazol-1-yl]phosphonate (2:1) (salt). | ||
Its empirical formula is C23H22F7N4O6P ∙ 2(C7H17NO5) and its structural formula is: | |||
[[File:XXXXX.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
|PD=There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |PD=There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | ||
Revision as of 14:04, 21 April 2015
{{DrugProjectFormSinglePage |authorTag=Kiran Singh, M.D. [1] |aOrAn=a |indicationType=treatment |adverseReactions= |blackBoxWarningTitle=ConditionName: |blackBoxWarningBody=ConditionName:
- Content
|fdaLIADAdult===Indications==
- EMEND® for Injection is a substance P/neurokinin-1 (NK1) receptor antagonist indicated in adults for use in combination with other antiemetic agents for the:
Limitations of Use
- Chronic continuous administration is not recommended
Dosage
Prevention of Nausea and Vomiting Associated with Highly Emetogenic Chemotherapy (HEC)
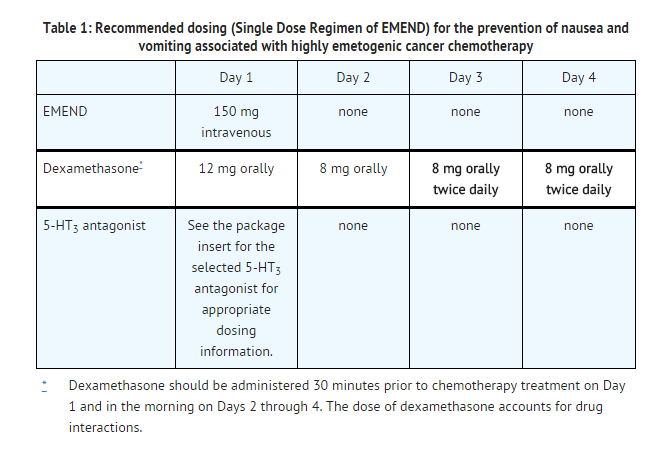
EMEND for Injection 150 mg (Single Dose Regimen of EMEND):
- EMEND for Injection 150 mg is administered intravenously on Day 1 only as an infusion over 20-30 minutes initiated approximately 30 minutes prior to chemotherapy. No capsules of EMEND are administered on Days 2 and 3. EMEND for Injection should be administered in conjunction with a corticosteroid and a 5-HT3 antagonist as specified in Table 1. The recommended dosage of dexamethasone with EMEND for Injection 150 mg differs from the recommended dosage of dexamethasone with EMEND for Injection 115 mg on Days 3 and 4. The package insert for the co-administered 5-HT3 antagonist must be consulted prior to initiation of treatment with EMEND for Injection.
EMEND for Injection 115 mg (3-Day Dosing Regimen of EMEND):
- EMEND for Injection 115 mg is administered on Day 1 only as an infusion over 15 minutes initiated 30 minutes prior to chemotherapy. Capsules of EMEND 80 mg should be administered on Days 2 and 3. EMEND for Injection 115 mg should be administered in conjunction with a corticosteroid and a 5-HT3 antagonist as specified in Table 2. The recommended dosage of dexamethasone with EMEND for Injection 115 mg differs from the recommended dosage of dexamethasone with EMEND for Injection 150 mg on Days 3 and 4. The package insert for the co-administered 5-HT3 antagonist must be consulted prior to initiation of treatment with EMEND for Injection.
- Capsules of EMEND 125 mg may be substituted for EMEND for Injection 115 mg on Day 1.

Prevention of Nausea and Vomiting Associated with Moderately Emetogenic Chemotherapy (MEC)
EMEND for Injection 115 mg (3-Day Dosing Regimen of EMEND):
- EMEND for Injection 115 mg is administered on Day 1 only as an infusion over 15 minutes initiated 30 minutes prior to chemotherapy. Capsules of EMEND 80 mg should be administered on Days 2 and 3. EMEND for Injection 115 mg should be administered in conjunction with a corticosteroid and a 5-HT3 antagonist as specified in Table 3. The recommended dosage of dexamethasone with EMEND for Injection 115 mg differs from the recommended dosage of dexamethasone with EMEND for Injection 150 mg on Days 3 and 4. The package insert for the co-administered 5-HT3 antagonist must be consulted prior to initiation of treatment with EMEND for Injection.
- Capsules of EMEND 125 mg may be substituted for EMEND for Injection 115 mg on Day 1.

Preparation of EMEND for Injection

- The reconstituted final drug solution is stable for 24 hours at ambient room temperature (at or below 25°C).
- Parenteral drug products should be inspected visually for particulate matter and discoloration before administration whenever solution and container permit.
- Caution: EMEND for Injection should not be mixed or reconstituted with solutions for which physical and chemical compatibility have not been established. EMEND for Injection is incompatible with any solutions containing divalent cations (e.g., Ca2+, Mg2+), including Lactated Ringer's Solution and Hartmann's Solution.
DOSAGE FORMS AND STRENGTHS
- One 150-mg single dose glass vial: White to off-white lyophilized solid (Sterile lyophilized powder for intravenous use only after reconstitution and dilution).
- One 115-mg single dose glass vial: White to off-white lyophilized solid (Sterile lyophilized powder for intravenous use only after reconstitution and dilution).
|offLabelAdultGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Fosaprepitant in adult patients. |offLabelAdultNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Fosaprepitant in adult patients. |fdaLIADPed=There is limited information regarding FDA-Labeled Use of Fosaprepitant in pediatric patients. |offLabelPedGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Fosaprepitant in pediatric patients. |offLabelPedNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Fosaprepitant in pediatric patients. |contraindications=Hypersensitivity
- EMEND for Injection is contraindicated in patients who are hypersensitive to EMEND for Injection, aprepitant, polysorbate 80 or any other components of the product. Known hypersensitivity reactions include: flushing, erythema, dyspnea, and anaphylactic reactions.
Concomitant Use with Pimozide or Cisapride
- Aprepitant, when administered orally, is a moderate cytochrome P450 isoenzyme 3A4 (CYP3A4) inhibitor following the 3-day antiemetic dosing regimen for CINV. Since fosaprepitant is rapidly converted to aprepitant, do not use fosaprepitant concurrently with pimozide or cisapride. Inhibition of CYP3A4 by aprepitant could result in elevated plasma concentrations of these drugs, potentially causing serious or life-threatening reactions.
|warnings=CYP3A4 Interactions
- Fosaprepitant is rapidly converted to aprepitant, which is a moderate inhibitor of CYP3A4 when administered as a 3-day antiemetic dosing regimen for CINV. Fosaprepitant should be used with caution in patients receiving concomitant medications that are primarily metabolized through CYP3A4. Inhibition of CYP3A4 by aprepitant or fosaprepitant could result in elevated plasma concentrations of these concomitant medications. When fosaprepitant is used concomitantly with another CYP3A4 inhibitor, aprepitant plasma concentrations could be elevated. When aprepitant is used concomitantly with medications that induce CYP3A4 activity, aprepitant plasma concentrations could be reduced, and this may result in decreased efficacy of aprepitant.
- Chemotherapy agents that are known to be metabolized by CYP3A4 include docetaxel, paclitaxel, etoposide, irinotecan, ifosfamide, imatinib, vinorelbine, vinblastine and vincristine. In clinical studies, the oral aprepitant regimen was administered commonly with etoposide, vinorelbine, or paclitaxel. The doses of these agents were not adjusted to account for potential drug interactions.
- In separate pharmacokinetic studies, no clinically significant change in docetaxel or vinorelbine pharmacokinetics was observed when the oral aprepitant regimen was coadministered.
- Due to the small number of patients in clinical studies who received the CYP3A4 substrates vinblastine, vincristine, or ifosfamide, particular caution and careful monitoring are advised in patients receiving these agents or other chemotherapy agents metabolized primarily by CYP3A4 that were not studied [see DRUG INTERACTIONS (7.1)].
Hypersensitivity Reactions
- Isolated reports of immediate hypersensitivity reactions including flushing, erythema, dyspnea, and anaphylaxis have occurred during infusion of fosaprepitant. These hypersensitivity reactions have generally responded to discontinuation of the infusion and administration of appropriate therapy. Reinitiation of the infusion is not recommended in patients who experience these symptoms during first-time use.
Coadministration with Warfarin (a CYP2C9 substrate)
- Coadministration of fosaprepitant or aprepitant with warfarin may result in a clinically significant decrease in International Normalized Ratio (INR) of prothrombin time. In patients on chronic warfarin therapy, the INR should be closely monitored in the 2-week period, particularly at 7 to 10 days, following initiation of fosaprepitant with each chemotherapy cycle.
Coadministration with Hormonal Contraceptives
- Upon coadministration with fosaprepitant or aprepitant, the efficacy of hormonal contraceptives may be reduced during and for 28 days following the last dose of either fosaprepitant or aprepitant. Alternative or back-up methods of contraception should be used during treatment with and for 1 month following the last dose of fosaprepitant or aprepitant.
Chronic Continuous Use
- Chronic continuous use of EMEND for Injection for prevention of nausea and vomiting is not recommended because it has not been studied; and because the drug interaction profile may change during chronic continuous use.
|clinicalTrials=Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
- Since EMEND for Injection is converted to aprepitant, those adverse reactions associated with aprepitant might also be expected to occur with EMEND for Injection.
- The overall safety of fosaprepitant was evaluated in approximately 1100 individuals and the overall safety of aprepitant was evaluated in approximately 6500 individuals.
Oral Aprepitant
Highly Emetogenic Chemotherapy (HEC)
- In 2 well-controlled clinical trials in patients receiving highly emetogenic cancer chemotherapy, 544 patients were treated with aprepitant during Cycle 1 of chemotherapy and 413 of these patients continued into the Multiple-Cycle extension for up to 6 cycles of chemotherapy. Oral aprepitant was given in combination with ondansetron and dexamethasone.
- In Cycle 1, adverse reactions were reported in approximately 17% of patients treated with the aprepitant regimen compared with approximately 13% of patients treated with standard therapy. Treatment was discontinued due to adverse reactions in 0.6% of patients treated with the aprepitant regimen compared with 0.4% of patients treated with standard therapy.
- The most common adverse reactions reported in patients treated with the aprepitant regimen with an incidence ≥1% and greater than standard therapy are listed in Table 5.
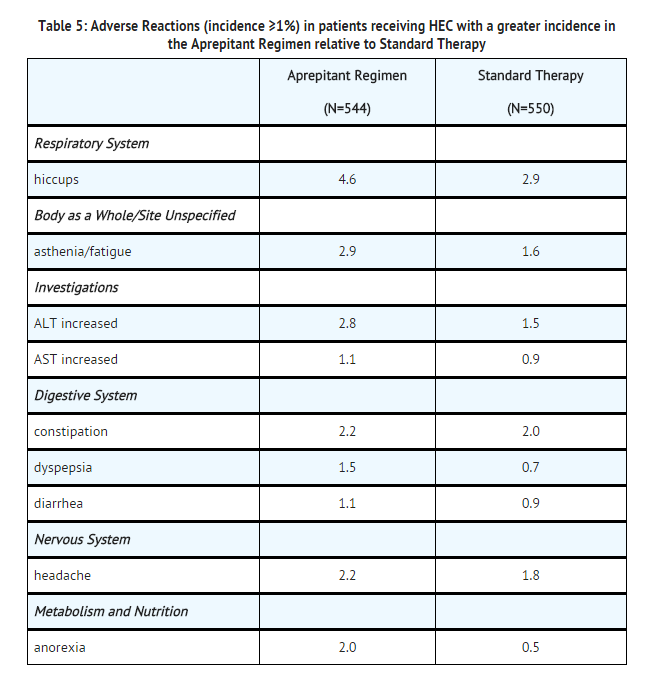
- A listing of adverse reactions in the aprepitant regimen (incidence <1%) that occurred at a greater incidence than standard therapy are presented in the Less Common Adverse Reactions subsection below.
- In an additional active-controlled clinical study in 1169 patients receiving aprepitant and highly emetogenic chemotherapy, the adverse experience profile was generally similar to that seen in the other HEC studies with aprepitant.
Moderately Emetogenic Chemotherapy (MEC)
- In 2 well-controlled clinical trials in patients receiving moderately emetogenic cancer chemotherapy, 868 patients were treated with the aprepitant regimen during Cycle 1 of chemotherapy and 686 of these patients continued into extensions for up to 4 cycles of chemotherapy. In both studies, oral aprepitant was given in combination with ondansetron and dexamethasone (aprepitant regimen).
- In the combined analysis of Cycle 1 data for these 2 studies, adverse reactions were reported in approximately 14% of patients treated with the aprepitant regimen compared with approximately 15% of patients treated with standard therapy. Treatment was discontinued due to adverse reactions in 0.7% of patients treated with the aprepitant regimen compared with 0.2% of patients treated with standard therapy.
- The most common adverse reactions reported in patients treated with the aprepitant regimen with an incidence ≥1% and greater than standard therapy are listed in Table 6.

- A listing of adverse reactions in the aprepitant regimen (incidence <1%) that occurred at a greater incidence than standard therapy are presented in the Less Common Adverse Reactions subsection below.
Less Common Adverse Reactions
Adverse reactions reported in either HEC or MEC studies in patients treated with the * aprepitant regimen with an incidence <1% and greater than standard therapy are listed in Table 7.

- In another chemotherapy induced nausea and vomiting (CINV) study, Stevens-Johnson syndrome was reported as a serious adverse reaction in a patient receiving aprepitant with cancer chemotherapy.
- The adverse experience profiles in the Multiple-Cycle extensions of HEC and MEC studies for up to 6 cycles of chemotherapy were similar to that observed in Cycle 1.
Fosaprepitant
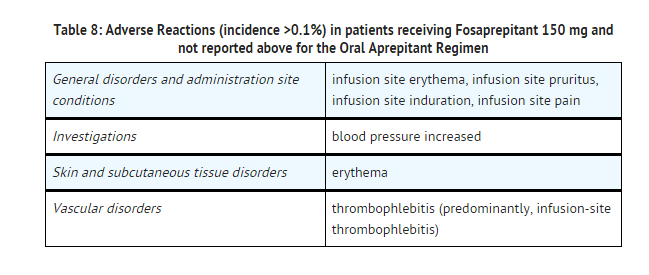
- In an active-controlled clinical study in patients receiving highly emetogenic chemotherapy, safety was evaluated for 1143 patients receiving the 1-day regimen of EMEND for Injection 150 mg compared to 1169 patients receiving the 3-day regimen of EMEND (aprepitant). The safety profile was generally similar to that seen in prior HEC studies with aprepitant. However, infusion-site reactions occurred at a higher incidence in patients in the fosaprepitant group (3.0%) compared to those in the aprepitant group (0.5%). The reported infusion-site reactions included infusion-site erythema, infusion-site pruritus, infusion-site pain, infusion-site induration, and infusion-site thrombophlebitis.
- The following additional adverse reactions occurred with fosaprepitant 150 mg and were not reported with the oral aprepitant regimen in the corresponding section above.
Other Studies with Postoperative Nausea and Vomiting
- In well-controlled clinical studies in patients receiving general balanced anesthesia, 564 patients were administered 40-mg aprepitant orally and 538 patients were administered 4-mg ondansetron intravenously.
- Adverse reactions were reported in approximately 4% of patients treated with 40-mg aprepitant compared with approximately 6% of patients treated with 4-mg ondansetron intravenously.
- In patients treated with aprepitant, increased ALT (1.1%) was seen at a greater incidence than with ondansetron (1.0%). The following additional adverse reactions were observed in patients treated with aprepitant at an incidence <1% and greater than with ondansetron.

n addition, two serious adverse reactions were reported in postoperative nausea and vomiting (PONV) clinical studies in patients taking a higher dose of aprepitant: one case of constipation, and one case of subileus.
Other Studies
- Angioedema and urticaria were reported as serious adverse reactions in a patient receiving aprepitant in a non-CINV/non-PONV study.
|postmarketing=* The following adverse reactions have been identified during post-approval use of fosaprepitant and aprepitant. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Skin and subcutaneous tissue disorders: pruritus, rash, urticaria, rarely Stevens-Johnson syndrome/toxic epidermal necrolysis.
- Immune system disorders: hypersensitivity reactions including anaphylactic reactions.
- Nervous system disorders: Events of ifosfamide-induced neurotoxicity have been reported after aprepitant and ifosfamide coadministration.
|drugInteractions=* Drug interactions following administration of fosaprepitant are likely to occur with drugs that interact with oral aprepitant.
- Aprepitant is a substrate, a moderate inhibitor, and an inducer of CYP3A4 when administered as a 3-day antiemetic dosing regimen for CINV. Aprepitant is also an inducer of CYP2C9.
- Fosaprepitant 150 mg, given as a single dose, is a weak inhibitor of CYP3A4, and does not induce CYP3A4. Fosaprepitant or aprepitant is unlikely to interact with drugs that are substrates for the P-glycoprotein transporter.
- The following information was derived from data with oral aprepitant, two studies conducted with fosaprepitant and oral midazolam, and one study conducted with fosaprepitant and dexamethasone.
Effect of Fosaprepitant/Aprepitant on the Pharmacokinetics of Other Agents CYP3A4 substrates:
- Aprepitant, as a moderate inhibitor of CYP3A4, and fosaprepitant 150 mg, as a weak inhibitor of CYP3A4, can increase plasma concentrations of concomitantly coadministered oral medications that are metabolized through CYP3A4.
5-HT3 antagonists:
- In clinical drug interaction studies, aprepitant did not have clinically important effects on the pharmacokinetics of ondansetron, granisetron, or hydrodolasetron (the active metabolite of dolasetron).
Corticosteroids:
- Dexamethasone: Fosaprepitant 150 mg administered as a single intravenous dose on Day 1 increased the AUC0-24hr of dexamethasone, administered as a single 8-mg oral dose on Days 1, 2, and 3, by approximately 2-fold on Days 1 and 2. The oral dexamethasone dose on Days 1 and 2 should be reduced by approximately 50% when coadministered with fosaprepitant 150-mg intravenous on Day 1.
- An oral aprepitant regimen of 125 mg on Day 1, and 80 mg/day on Days 2 through 5, coadministered with 20-mg oral dexamethasone on Day 1 and 8-mg oral dexamethasone on Days 2 through 5, increased the AUC of dexamethasone by 2.2-fold on Days 1 and 5. The oral dexamethasone doses should be reduced by approximately 50% when coadministered with a regimen of fosaprepitant 115 mg followed by aprepitant.
- Methylprednisolone: An oral aprepitant regimen of 125 mg on Day 1 and 80 mg/day on Days 2 and 3 increased the AUC of methylprednisolone by 1.34-fold on Day 1 and by 2.5-fold on Day 3, when methylprednisolone was coadministered intravenously as 125 mg on Day 1 and orally as 40 mg on Days 2 and 3. The intravenous methylprednisolone dose should be reduced by approximately 25%, and the oral methylprednisolone dose should be reduced by approximately 50% when coadministered with a regimen of fosaprepitant 115 mg followed by aprepitant.
Chemotherapeutic agents:
- Docetaxel: In a pharmacokinetic study, oral aprepitant (CINV regimen) did not influence the pharmacokinetics of docetaxel.
- Vinorelbine: In a pharmacokinetic study, oral aprepitant (CINV regimen) did not influence the pharmacokinetics of vinorelbine to a clinically significant degree.
- Other Chemotherapeutic Agents: EMEND for Injection should be used with caution in patients receiving other chemotherapeutic agents that are primarily metabolized through CYP3A4.
Oral contraceptives:
- When oral aprepitant, ondansetron, and dexamethasone were coadministered with an oral contraceptive containing ethinyl estradiol and norethindrone, the trough concentrations of both ethinyl estradiol and norethindrone were reduced by as much as 64% for 3 weeks post-treatment.
- The coadministration of fosaprepitant or aprepitant may reduce the efficacy of hormonal contraceptives (these can include birth control pills, skin patches, implants, and certain IUDs) during and for 28 days after administration of the last dose of fosaprepitant or aprepitant. Alternative or back-up methods of contraception should be used during treatment with and for 1 month following the last dose of fosaprepitant or aprepitant.
Midazolam:
- Interactions between aprepitant or fosaprepitant and coadministered midazolam are listed in the table below (increase is indicated as "↑", decrease as "↓", no change as "↔").

- A difference of less than 2-fold increase of midazolam AUC was not considered clinically important.
- The potential effects of increased plasma concentrations of midazolam or other benzodiazepines metabolized via CYP3A4 (alprazolam, triazolam) should be considered when coadministering these agents with fosaprepitant or aprepitant.
CYP2C9 substrates (Warfarin, Tolbutamide):
- Warfarin: A single 125-mg dose of oral aprepitant was administered on Day 1 and 80 mg/day on Days 2 and 3 to healthy subjects who were stabilized on chronic warfarin therapy. Although there was no effect of oral aprepitant on the plasma AUC of R(+) or S(-) warfarin determined on Day 3, there was a 34% decrease in S(-) warfarin trough concentration accompanied by a 14% decrease in the prothrombin time (reported as International Normalized Ratio or INR) 5 days after completion of dosing with oral aprepitant. In patients on chronic warfarin therapy, the prothrombin time (INR) should be closely monitored in the 2-week period, particularly at 7 to 10 days, following initiation of fosaprepitant with each chemotherapy cycle.
- Tolbutamide: Oral aprepitant, when given as 125 mg on Day 1 and 80 mg/day on Days 2 and 3, decreased the AUC of tolbutamide by 23% on Day 4, 28% on Day 8, and 15% on Day 15, when a single dose of tolbutamide 500 mg was administered orally prior to the administration of the 3-day regimen of oral aprepitant and on Days 4, 8, and 15.
Effect of Other Agents on the Pharmacokinetics of Aprepitant
- Aprepitant is a substrate for CYP3A4; therefore, coadministration of fosaprepitant or aprepitant with drugs that inhibit CYP3A4 activity may result in increased plasma concentrations of aprepitant. Consequently, concomitant administration of fosaprepitant or aprepitant with strong CYP3A4 inhibitors (e.g., ketoconazole, itraconazole, nefazodone, troleandomycin, clarithromycin, ritonavir, nelfinavir) should be approached with caution. Because moderate CYP3A4 inhibitors (e.g., diltiazem) result in a 2-fold increase in plasma concentrations of aprepitant, concomitant administration should also be approached with caution.
- Aprepitant is a substrate for CYP3A4; therefore, coadministration of fosaprepitant or aprepitant with drugs that strongly induce CYP3A4 activity (e.g., rifampin, carbamazepine, phenytoin) may result in reduced plasma concentrations and decreased efficacy.
- Ketoconazole: When a single 125-mg dose of oral aprepitant was administered on Day 5 of a 10-day regimen of 400 mg/day of ketoconazole, a strong CYP3A4 inhibitor, the AUC of aprepitant increased approximately 5-fold and the mean terminal half-life of aprepitant increased approximately 3-fold. Concomitant administration of fosaprepitant or aprepitant with strong CYP3A4 inhibitors should be approached cautiously.
- Rifampin: When a single 375-mg dose of oral aprepitant was administered on Day 9 of a 14-day regimen of 600 mg/day of rifampin, a strong CYP3A4 inducer, the AUC of aprepitant decreased approximately 11-fold and the mean terminal half-life decreased approximately 3-fold.
- Coadministration of fosaprepitant or aprepitant with drugs that induce CYP3A4 activity may result in reduced plasma concentrations and decreased efficacy.
Additional Interactions
Diltiazem:
- In a study in 10 patients with mild to moderate hypertension, intravenous infusion of 100 mg of fosaprepitant with diltiazem 120 mg 3 times daily, resulted in a 1.5-fold increase of aprepitant AUC and a 1.4-fold increase in diltiazem AUC. It also resulted in a small but clinically meaningful further maximum decrease in diastolic blood pressure [mean (SD) of 24.3 (± 10.2) mm Hg with fosaprepitant versus 15.6 (± 4.1) mm Hg without fosaprepitant] and resulted in a small further maximum decrease in systolic blood pressure [mean (SD) of 29.5 (± 7.9) mm Hg with fosaprepitant versus 23.8 (± 4.8) mm Hg without fosaprepitant], which may be clinically meaningful, but did not result in a clinically meaningful further change in heart rate or PR interval, beyond those changes induced by diltiazem alone.
- In the same study, administration of aprepitant once daily, as a tablet formulation comparable to 230 mg of the capsule formulation, with diltiazem 120 mg 3 times daily for 5 days, resulted in a 2-fold increase of aprepitant AUC and a simultaneous 1.7-fold increase of diltiazem AUC. These pharmacokinetic effects did not result in clinically meaningful changes in ECG, heart rate or blood pressure beyond those changes induced by diltiazem alone.
Paroxetine:
- Coadministration of once daily doses of aprepitant, as a tablet formulation comparable to 85 mg or 170 mg of the capsule formulation, with paroxetine 20 mg once daily, resulted in a decrease in AUC by approximately 25% and Cmax by approximately 20% of both aprepitant and paroxetine.
|useInPregnancyFDA=Teratogenic effects
Pregnancy Category B: In the reproduction studies conducted with fosaprepitant and aprepitant, the highest systemic exposures to aprepitant were obtained following oral administration of aprepitant. Reproduction studies performed in rats at oral doses of aprepitant up to 1000 mg/kg twice daily (plasma AUC0-24hr of 31.3 mcg•hr/mL, about 1.6 times the human exposure at the recommended dose) and in rabbits at oral doses up to 25 mg/kg/day (plasma AUC0-24hr of 26.9 mcg•hr/mL, about 1.4 times the human exposure at the recommended dose) revealed no evidence of impaired fertility or harm to the fetus due to aprepitant. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. |useInPregnancyAUS=* Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Fosaprepitant in women who are pregnant. |useInLaborDelivery=There is no FDA guidance on use of Fosaprepitant during labor and delivery. |useInNursing=* Aprepitant is excreted in the milk of rats. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for possible serious adverse reactions in nursing infants from aprepitant and because of the potential for tumorigenicity shown for aprepitant in rodent carcinogenicity studies, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. |useInPed=* Safety and effectiveness of EMEND for Injection in pediatric patients have not been established. |useInGeri=* In 2 well-controlled chemotherapy-induced nausea and vomiting clinical studies, of the total number of patients (N=544) treated with oral aprepitant, 31% were 65 and over, while 5% were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects. Greater sensitivity of some older individuals cannot be ruled out. Dosage adjustment in the elderly is not necessary |useInGender=There is no FDA guidance on the use of Fosaprepitant with respect to specific gender populations. |useInRace=There is no FDA guidance on the use of Fosaprepitant with respect to specific racial populations. |useInRenalImpair=There is no FDA guidance on the use of Fosaprepitant in patients with renal impairment. |useInHepaticImpair=* There are no clinical or pharmacokinetic data in patients with severe hepatic impairment (Child-Pugh score >9). Therefore, caution should be exercised when fosaprepitant or aprepitant is administered in these patients |useInReproPotential=There is no FDA guidance on the use of Fosaprepitant in women of reproductive potentials and males. |useInImmunocomp=There is no FDA guidance one the use of Fosaprepitant in patients who are immunocompromised.
|administration=* Oral
- Intravenous
|monitoring=There is limited information regarding Monitoring of Fosaprepitant in the drug label.
- Description
|IVCompat=There is limited information regarding IV Compatibility of Fosaprepitant in the drug label.
|overdose=* There is no specific information on the treatment of overdosage with fosaprepitant or aprepitant.
- In the event of overdose, fosaprepitant and/or oral aprepitant should be discontinued and general supportive treatment and monitoring should be provided. Because of the antiemetic activity of aprepitant, drug-induced emesis may not be effective.
- Aprepitant cannot be removed by hemodialysis.
- Thirteen patients in the randomized controlled trial of EMEND for Injection received both fosaprepitant 150 mg and at least one dose of oral aprepitant, 125 mg or 80 mg. Three patients reported adverse reactions that were similar to those experienced by the total study population.
|drugBox= |mechAction=*
|structure=EMEND (fosaprepitant dimeglumine) for Injection is a sterile, lyophilized prodrug of aprepitant, a substance P/neurokinin-1 (NK1) receptor antagonist, and is chemically described as 1-Deoxy-1-(methylamino)-D-glucitol[3-[[(2R,3S)-2-[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3-(4-fluorophenyl)-4-morpholinyl]methyl]-2,5-dihydro-5-oxo-1H-1,2,4-triazol-1-yl]phosphonate (2:1) (salt).
Its empirical formula is C23H22F7N4O6P ∙ 2(C7H17NO5) and its structural formula is:
|PD=There is limited information regarding Pharmacodynamics of Fosaprepitant in the drug label.
|PK=There is limited information regarding Pharmacokinetics of Fosaprepitant in the drug label.
|nonClinToxic=There is limited information regarding Nonclinical Toxicology of Fosaprepitant in the drug label.
|clinicalStudies=There is limited information regarding Clinical Studies of Fosaprepitant in the drug label.
|howSupplied=* |packLabel= |fdaPatientInfo=There is limited information regarding Patient Counseling Information of Fosaprepitant in the drug label.
|alcohol=* Alcohol-Fosaprepitant interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
|brandNames=* EMEND ®[1] |lookAlike=* A® — B®[2] |drugShortage= }} {{#subobject:
|Page Name=Fosaprepitant
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Fosaprepitant |Label Name=Fosaprepitant11.png
}}
{{#subobject:
|Label Page=Fosaprepitant |Label Name=Fosaprepitant11.png
}}
- ↑ "fosaprepitant dimeglumine injection, powder, lyophilized, for solution".
- ↑ "http://www.ismp.org". External link in
|title=(help)