Flurbiprofen (ophthalmic)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Adeel Jamil, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Flurbiprofen (ophthalmic) is an analgesic, NSAID and propionic acid that is FDA approved for the treatment of inhibition of intraoperative miosis. Common adverse reactions include abnormal vision, transient burning, stinging and ocular irritation.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Flurbiprofen sodium ophthalmic solution is indicated for the inhibition of intraoperative miosis.
Dosing Information
- A total of four (4) drops of flurbiprofen sodium ophthalmic solution should be administered by instilling 1 drop approximately every 1/2 hour beginning 2 hours before surgery.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Flurbiprofen (ophthalmic) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Flurbiprofen (ophthalmic) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Flurbiprofen (ophthalmic) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Flurbiprofen (ophthalmic) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Flurbiprofen (ophthalmic) in pediatric patients.
Contraindications
- Flurbiprofen sodium ophthalmic solution is contraindicated in individuals who are hypersensitive to any components of the medication.
Warnings
- With some nonsteroidal anti-inflammatory drugs, there exists the potential for increased bleeding due to interference with thrombocyte aggregation. There have been reports that flurbiprofen sodium ophthalmic solution may cause increased bleeding of ocular tissues (including hyphemas) in conjunction with ocular surgery.
- There is the potential for cross-sensitivity to acetylsalicylic acid and other nonsteroidal anti-inflammatory drugs. Therefore, caution should be used when treating individuals who have previously exhibited sensitivities to these drugs.
PRECAUTIONS
General:
- Topical nonsteroidal anti-inflammatory drugs (NSAIDs) may slow or delay healing. Topical corticosteroids are also known to slow or delay healing. Concomitant use of topical NSAIDs and topical steroids may increase the potential for healing problems.
- It is recommended that flurbiprofen sodium ophthalmic solution be used with caution in surgical patients with known bleeding tendencies or who are receiving other medications which may prolong bleeding time.
Adverse Reactions
Clinical Trials Experience
- Transient burning and stinging upon instillation and other minor symptoms of ocular irritation have been reported with the use of flurbiprofen sodium ophthalmic solution. Other adverse reactions reported with the use of flurbiprofen sodium ophthalmic solution include: fibrosis, hyphema, miosis, mydriasis, and ocular hyeremia.
- Increased bleeding tendency of ocular tissues in conjunction with ocular surgery has also been reported.
Postmarketing Experience
There is limited information regarding Flurbiprofen (ophthalmic) Postmarketing Experience in the drug label.
Drug Interactions
- Interaction of flurbiprofen sodium ophthalmic solution with other topical ophthalmic medications has not been fully investigated.
- Although clinical studies with acetylcholine chloride and animal studies with acetylcholine chloride or carbachol revealed no interference, and there is no known pharmacological basis for an interaction, there have been reports that acetylcholine chloride and carbachol have been ineffective when used in patients treated with flurbiprofen sodium ophthalmic solution.
Use in Specific Populations
Pregnancy
- Flurbiprofen has been shown to be embryocidal, delay parturition, prolong gestation, reduce weight, and/or slightly retard growth of fetuses when given to rats in daily oral doses of 0.4 mg/kg (approximately 300 times the human daily topical dose) and above. There are no adequate and well-controlled studies in pregnant women. Flurbiprofen sodium ophthalmic solution should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Flurbiprofen (ophthalmic) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Flurbiprofen (ophthalmic) during labor and delivery.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from flurbiprofen sodium, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
There is no FDA guidance on the use of Flurbiprofen (ophthalmic) in pediatric settings.
Geriatic Use
- No overall differences in safety or effectiveness have been observed between elderly and younger patients.
Gender
There is no FDA guidance on the use of Flurbiprofen (ophthalmic) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Flurbiprofen (ophthalmic) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Flurbiprofen (ophthalmic) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Flurbiprofen (ophthalmic) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Flurbiprofen (ophthalmic) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Flurbiprofen (ophthalmic) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Ophthalmic
Monitoring
There is limited information regarding Flurbiprofen (ophthalmic) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Flurbiprofen (ophthalmic) and IV administrations.
Overdosage
- Overdosage will not ordinarily cause acute problems. If accidentally ingested, drink fluids to dilute.
Pharmacology
Mechanism of Action
- Flurbiprofen sodium is one of a series of phenylalkanoic acids that have shown analgesic antipyretic, and antiinflammatory activity in animal inflammatory diseases. Its mechanism of action is believed to be through inhibition of the cyclo-oxygenase enzyme that is essential in the biosynthesis of prostaglandins.
- Prostaglandins have been shown in many animal models to be mediators of certain kinds of intraocular inflammation. In studies performed on animal eyes, prostaglandins have been shown to produce disruption of the blood-aqueous humor barrier, vasodilatation, increased vascular permeability, leukocytosis, and increased intraocular pressure.
- Prostaglandins also appear to play a role in the miotic response produced during ocular surgery by constricting the iris sphincter independently of cholinergic mechanisms. In clinical studies, flurbiprofen sodium ophthalmic solution has been shown to inhibit the miosis induced during the course of cataract surgery.
- Results from clinical studies indicate that flurbiprofen sodium has no significant effect upon intraocular pressure.
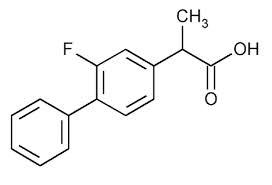
Structure
- Flurbiprofen Sodium Ophthalmic Solution USP, 0.03% is a sterile topical nonsteroidal anti-inflammatory product for ophthalmic use.
- Chemical Name:
- Sodium (±)-2-(2-fluoro-4-biphenylyl) propionate dihydrate.
- Structural Formula:

Pharmacodynamics
There is limited information regarding Flurbiprofen (ophthalmic) Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Flurbiprofen (ophthalmic) Pharmacokinetics in the drug label.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility:
- Long-term studies in mice and/or rats have shown no evidence of carcinogenicity with flurbiprofen.
- Long-term mutagenicity studies in animals have not been performed.
Clinical Studies
There is limited information regarding Flurbiprofen (ophthalmic) Clinical Studies in the drug label.
How Supplied
Flurbiprofen Sodium Ophthalmic Solution USP, 0.03% is supplied in a plastic bottle with a controlled drop tip in the following size:
2.5 mL NDC 24208-314-25
DO NOT USE IF IMPRINTED “Protective Seal” WITH YELLOW symbol IS NOT INTACT.
Revised: November 2012 Bausch & Lomb Incorporated Tampa, Florida 33637 ©Bausch & Lomb Incorporated
Storage
- Store at 15°–25°C (59°–77°F).
Images
Drug Images
{{#ask: Page Name::Flurbiprofen (ophthalmic) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
NDC 24208-314-25
Bausch & Lomb
Flurbiprofen Sodium Ophthalmic Solution USP, 0.03% (Sterile)
Rx only
[icon- eye] [icon- 0.03%] [icon- solution] [icon- 2.5 mL]

{{#ask: Label Page::Flurbiprofen (ophthalmic) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Patients should be instructed to avoid allowing the tip of the bottle to contact the eye or surrounding structures because this could cause the tip to become contaminated by common bacteria known to cause ocular infections. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions.
- To avoid the potential for cross-contamination, the patient should be advised to use one bottle for each eye with bilateral ocular surgery. The use of the same bottle of eye drops for both eyes is not recommended with ocular surgery.
Precautions with Alcohol
Alcohol-Flurbiprofen (ophthalmic) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Ansaid
Look-Alike Drug Names
There is limited information regarding Flurbiprofen (ophthalmic) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.