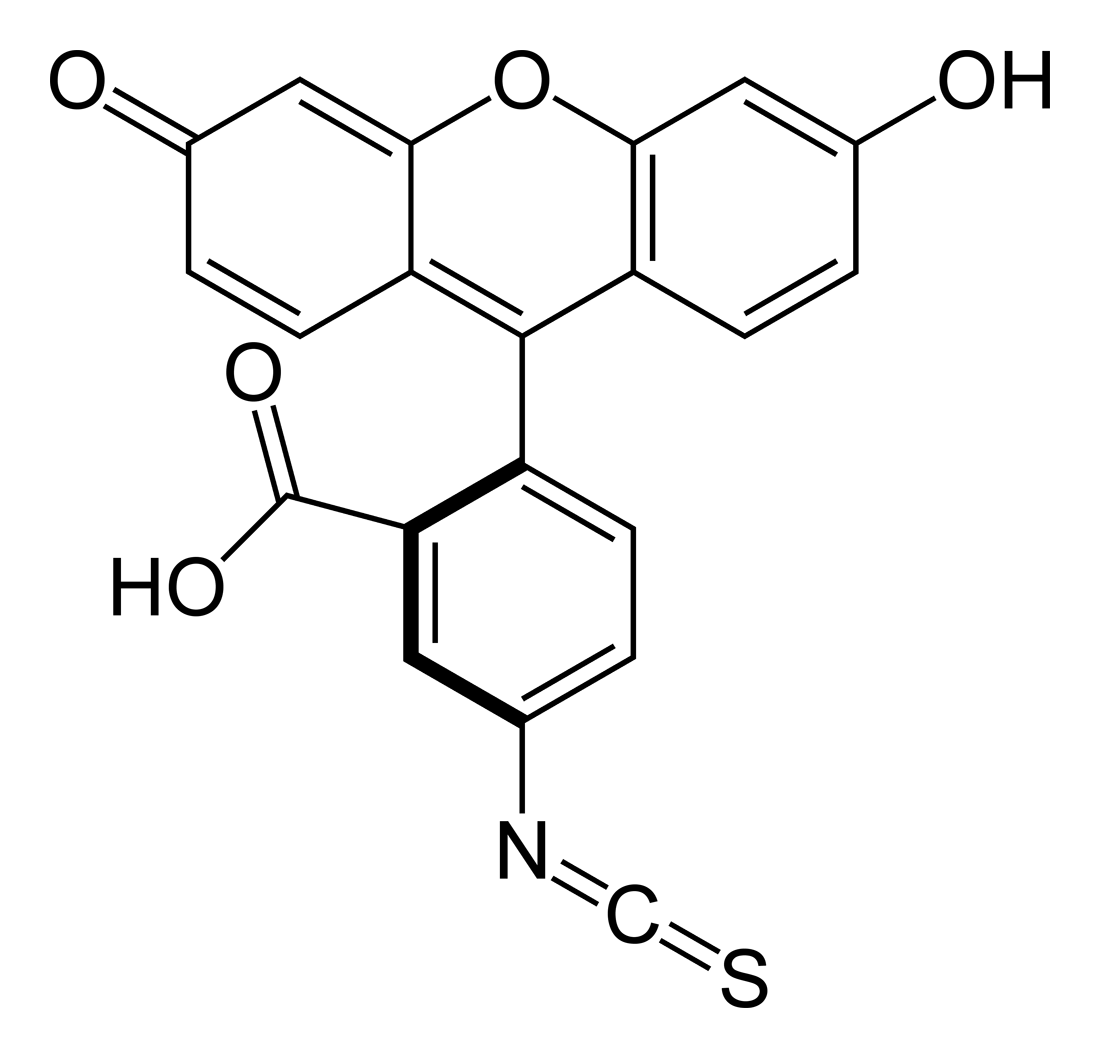
Fluorescein
|
WikiDoc Resources for Fluorescein |
|
Articles |
|---|
|
Most recent articles on Fluorescein Most cited articles on Fluorescein |
|
Media |
|
Powerpoint slides on Fluorescein |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Fluorescein at Clinical Trials.gov Clinical Trials on Fluorescein at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Fluorescein
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Fluorescein Discussion groups on Fluorescein Patient Handouts on Fluorescein Directions to Hospitals Treating Fluorescein Risk calculators and risk factors for Fluorescein
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Fluorescein |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Fluorescein is a fluorophore commonly used in microscopy, in a type of dye laser as the gain medium, in forensics and serology to detect latent blood stains, and in dye tracing. Fluorescein has an absorption maximum at 494 nm and emission maximum of 521 nm (in water). Also, fluorescein has an isosbestic point (equal absorption for all pH values) at 460 nm. Fluorescein is also known as a color additive (D&C Yellow no. 7). The disodium salt form of fluorescein is known as D&C Yellow no. 8.
Chemical and physical properties
The fluorescence of this molecule is very high, and excitation occurs at 494 nm and emission at 521.
Fluorescein has a pKa of 6.4 and its ionization equilibrium leads to pH-dependent absorption and emission over the range of 5 to 9. Also, the fluorescence lifetimes of the protonated and deprotonated forms of fluorescein are approximately 3 and 4 ns, which allows for pH determination from non-intensity based measurements. The lifetimes can be recovered using time-correlated single photon counting or phase-modulation fluorimetry.
Derivatives
There are many fluorescein derivatives, for example fluorescein isothiocyanate, often abbreviated as FITC. FITC is the original fluorescein molecule functionalized with an isothiocyanate group (-N=C=S), replacing a hydrogen atom on the bottom ring of the structure. This derivative is reactive towards amine groups on proteins inside cells. A succinimidyl-ester functional group attached to the fluorescein core, creating NHS-fluorescein, forms another common amine reactive derivative.
Other derivatives of fluorescein include Oregon Green, Tokyo Green, SNAFL, and carboxynaphthofluorescein. These derivatives, along with newer fluors such as Alexa 488 and DyLight 488, have been tailored for various chemical and biological applications where higher photostability, different spectral characteristics, or different attachment groups are needed.
Synthesis
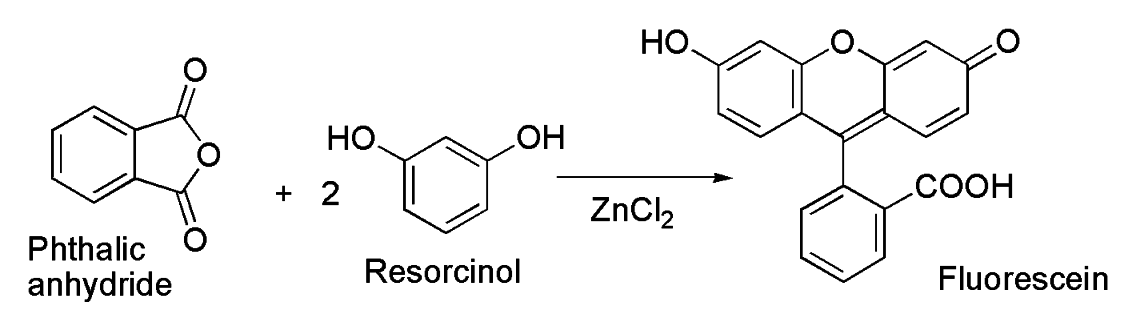
Fluorescein was first synthesized by Adolf von Baeyer in 1871. It can be prepared from phthalic anhydride and resorcinol in the presence of zinc chloride via the Friedel-Crafts reaction.
A second method to prepare fluorescein uses methanesulfonic acid as a Lewis acid catalyst. This route has a high yield under milder conditions. [1] [2]
Applications
Uses in river systems
One of its more recognizable uses was in the Chicago River, where fluorescein was the first substance used to dye the river green on St. Patrick's Day in 1962. In 1966 environmentalists forced a change to a vegetable based dye to protect the thousands of goldfish that populate the river.
Other uses of fluorescein include using it as a water-soluble dye added to rainwater in environmental testing simulations to aid in locating and analyzing any water leaks, and in Australia and New Zealand as a methylated spirit dye.
Biochemical research
In cellular biology, the isothiocyanate derivative of fluorescein is often used to label and track cells in fluorescence microscopy applications (for example, flow cytometry). Additional biologically active molecules (such as antibodies) may also be attached to fluorescein, allowing biologists to target the fluorophore to specific proteins or structures within cells. This application is common in yeast display.
Fluorescein can also be conjugated to nucleoside triphosphates and incorporated into a probe for in situ hybridisation. Fluorescein-labelled probes can be imaged using FISH, or targeted by antibodies using immunohistochemistry. The latter is a common alternative to digoxigenin, and the two are used together for labelling two genes in one sample [3].
Health care applications
Fluorescein sodium is used extensively as a diagnostic tool in the field of ophthalmology, where topical fluorescein is used in the diagnosis of corneal abrasions, corneal ulcers, herpetic corneal infections.
Intravenous or oral fluorescein is used in fluorescein angiography in research and to diagnose and categorize vascular disorders in e.g. legs, including retinal disease macular degeneration, diabetic retinopathy, inflammatory intraocular conditions, and intraocular tumors , and increasingly during surgery for brain tumors.
Safety
Topical, oral, and intravenous use of fluorescein can cause adverse reactions including nausea, vomiting, hives, acute hypotension, anaphylaxis and related anaphylactoid reaction,[4] cardiac arrest,[5] and sudden death.[6][7] Intravenous use has the most reported adverse reactions, including sudden death, but this may reflect greater use rather than greater risk. Both oral and topical uses have been reported to cause anaphylaxis,[8][9] including one case of anaphylaxis with cardiac arrest (resuscitated) following topical use in an eye drop.[5] Reported rates of adverse reactions vary from 1% to 6%[10][11][12][13] The higher rates may reflect study populations that include a higher percentage of persons with prior adverse reactions. The risk of an adverse reaction is 25 times higher if the person has had a prior adverse reaction.[12] The risk can be reduced with prior (prophylactic) use of antihistamines[14] and prompt emergency management of any ensuing anaphylaxis.[15] A simple prick test may help to identify persons at greatest risk of adverse reaction.[13]
The most common adverse reaction is nausea, due to a difference in the pH from the body and the pH of the sodium fluorescein dye. The nausea usually is transient and subsides quickly. Hives can range from a minor annoyance to severe. A single dose of antihistamine may give complete relief. Anaphylactic shock and subsequent cardiac arrest and sudden death are very rare but because they occur within minutes, a health care provider who uses fluorescein should be prepared to perform emergency resuscitation.
See also
- Chemical derivatives of fluorescein
- Eosin
- Fluorescein isothiocyanate
- Erythrosine
- DyLight Fluor, a product line of fluorescent dyes
- Pseudomonas aeruginosa, a bacterium that secretes fluorescein
- Fluorescein diacetate hydrolysis, a biochemistry laboratory test
References
- ↑ Sun, W. C.; Gee, K. R.; Klaubert, D. H.; Haugland, R. P., Synthesis of Fluorinated Fluoresceins. Journal of Organic Chemistry 1997, 62, (19), 6469-6475.
- ↑ Yuichiro Ueno; Jiao, G.-S.; Burgess, K., Preparation of 5- and 6-Carboxyfluorescein. Practical Synthetic Procedures 2004, 31, (15), 2591-2593.
- ↑ Noga E. J., Udomkusonsri, P. (2002). "Fluorescein: A Rapid, Sensitive, Nonlethal Method for Detecting Skin Ulceration in Fish" (PDF). Vet Pathol. 39: 726–731(6). doi:10.1354/vp.39-6-726. PMID 12450204. Retrieved 2007-07-16.
- ↑ The Diagnosis and Management of Anaphylaxis - XXI. Anaphylactoid reactions to fluorescein. J Allergy Clin Immunol 1998;101:S465-528. Free full text; see also The diagnosis and management of anaphylaxis: an updated practice parameter. from the National Guideline Clearinghouse.
- ↑ 5.0 5.1 el Harrar N, Idali B, Moutaouakkil S, el Belhadji M, Zaghloul K, Amraoui A, Benaguida M (1996). "[Anaphylactic shock caused by application of fluorescein on the ocular conjunctiva]". Presse Med (in French). 25 (32): 1546–7. PMID 8952662.
- ↑ Fineschi V, Monasterolo G, Rosi R, Turillazzi E (1999). "Fatal anaphylactic shock during a fluorescein angiography". Forensic Sci. Int. 100 (1–2): 137–42. doi:10.1016/S0379-0738(98)00205-9. PMID 10356782.
- ↑ Hitosugi M, Omura K, Yokoyama T, Kawato H, Motozawa Y, Nagai T, Tokudome S (2004). "An autopsy case of fatal anaphylactic shock following fluorescein angiography: a case report". Med Sci Law. 44 (3): 264–5. PMID 15296251.
- ↑ Kinsella FP, Mooney DJ (1988). "Anaphylaxis following oral fluorescein angiography". Am. J. Ophthalmol. 106 (6): 745–6. PMID 3195657.
- ↑ Gómez-Ulla F, Gutiérrez C, Seoane I (1991). "Severe anaphylactic reaction to orally administered fluorescein". Am. J. Ophthalmol. 112 (1): 94. PMID 1882930.
- ↑ Kwan AS, Barry C, McAllister IL, Constable I (2006). "Fluorescein angiography and adverse drug reactions revisited: the Lions Eye experience". Clin. Experiment. Ophthalmol. 34 (1): 33–8. doi:10.1111/j.1442-9071.2006.01136.x. PMID 16451256.
- ↑ Jennings BJ, Mathews DE (1994). "Adverse reactions during retinal fluorescein angiography". J Am Optom Assoc. 65 (7): 465–71. PMID 7930354.
- ↑ 12.0 12.1 Kwiterovich KA, Maguire MG, Murphy RP, Schachat AP, Bressler NM, Bressler SB, Fine SL (1991). "Frequency of adverse systemic reactions after fluorescein angiography. Results of a prospective study". Ophthalmology. 98 (7): 1139–42. PMID 1891225.
- ↑ 13.0 13.1 Matsuura M, Ando F, Fukumoto K, Kyogane I, Torii Y, Matsuura M (1996). "[Usefulness of the prick test for anaphylactoid reaction in intravenous fluorescein administration]". Nippon Ganka Gakkai Zasshi (in Japanese). 100 (4): 313–7. PMID 8644545.
- ↑ Ellis PP, Schoenberger M, Rendi MA (1980). "Antihistamines as prophylaxis against side reactions to intravenous fluorescein". Trans Am Ophthalmol Soc. 78: 190–205. PMID 7257056.
- ↑ Yang CS, Sung CS, Lee FL, Hsu WM (2007). "Management of anaphylactic shock during intravenous fluorescein angiography at an outpatient clinic". J Chin Med Assoc. 70 (8): 348–9. doi:10.1016/S1726-4901(08)70017-0. PMID 17698436.
External links
- Absorption and Emission Spectra of Fluorescein in Ethanol and Basic Ethanol at OGI School of Science and Engineering
- Fluorescein Ionization Equilibria at Invitrogen
- MSDS at Oxford University
- Absorption spectra and fluorescence emission spectra
ca:Fluoresceïna de:Fluorescein eo:Fluoreskeino sv:Fluorescein