Famotidine (oral): Difference between revisions
Kiran Singh (talk | contribs) No edit summary |
No edit summary |
||
| (24 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag={{KS}} | |authorTag={{KS}} | ||
|OTC=Yes | |||
|genericName=famotidine (oral) | |||
|aOrAn=a | |aOrAn=a | ||
| | |drugClass=gastric acid secretion inhibitor | ||
|adverseReactions= | |indicationType=treatment | ||
|indication=[[heartburn]],gastric hypersecretion, [[gastric ulcer]],[[gastroesophageal reflux]], | |||
[[indigestion]] | |||
|adverseReactions=[[constipation]] ,[[diarrhea]], [[dizziness]] , [[headache]] | |||
|blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | |blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | |blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | ||
| Line 13: | Line 18: | ||
<!--FDA-Labeled Indications and Dosage (Adult)--> | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
|fdaLIADAdult===Indications== | |fdaLIADAdult===Indications== | ||
* | * [[Duodenal ulcer]] | ||
* Esophagitis - [[gastroesophageal reflux]] disease, | |||
* Gastric hypersecretion | |||
* [[Gastric ulcer]] | |||
* [[Gastroesophageal reflux]] disease | |||
* [[Indigestion]] | |||
==Dosing== | |||
* | * To relieve symptoms, swallow 1 tablet with a glass of water. Do not chew. | ||
* | * To prevent symptoms, swallow 1 tablet with a glass of water at any time from 10 to 60 minutes before eating food or drinking beverages that cause heartburn | ||
* Do not use more than 2 tablets in 24 hours | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of famotidine in adult patients. | |||
|offLabelAdultNoGuideSupport===Indications== | |||
* Esophagitis, Maintenance | |||
* [[Gastritis]] | |||
* [[Gastrointestinal hemorrhage]] | |||
* [[Stress ulcer]] | |||
* [[Urticaria]] | |||
==Dosing== | |||
* Duodenal ulcer disease: acute, 40 mg orally once daily at bedtime or 20 mg orally twice daily | |||
* Duodenal ulcer disease: maintenance, 20 mg orally once daily at bedtime | |||
* | * Esophagitis - Gastroesophageal reflux disease, Short term treatment: 20 to 40 mg orally twice daily for up to 12 weeks | ||
: | * Gastric hypersecretion: 20 to 160 mg orally every 6 h. | ||
* Gastric ulcer, Short term treatment: acute, 40 mg orally once daily at bedtime | |||
* Gastroesophageal reflux disease, Short-term, symptom treatment: 20 mg orally twice daily for up to 6 wks | |||
* Indigestion: 10 to 20 mg orally twice daily | |||
|fdaLIADPed===Indication== | |||
* [[Duodenal ulcer]] | |||
* Esophagitis - [[gastroesophageal reflux]] disease, | |||
* Gastric hypersecretion | |||
* [[Gastric ulcer]] | |||
* [[Gastroesophageal reflux]] disease | |||
* [[Indigestion]] | |||
==Dosing== | |||
: | '''Children 12 years and over''': | ||
* To relieve symptoms, swallow 1 tablet with a glass of water. Do not chew. | |||
* To prevent symptoms, swallow 1 tablet with a glass of water at any time from 10 to 60 minutes before eating food or drinking beverages that cause heartburn | |||
* Do not use more than 2 tablets in 24 hours | |||
< | Children under 12 years: ask a doctor | ||
| | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Famotidine in pediatric patients. | ||
|offLabelPedNoGuideSupport===Indications== | |||
* [[Stress ulcer]]; Prophylaxis | |||
* | ==Dosage== | ||
* Duodenal ulcer disease: (over 1 y old) 0.5 mg/kg/day orally at bedtime or divided twice daily; max dose 40 mg/day | |||
* | * Gastric ulcer, Short term treatment: (over 1 y old) 0.5 mg/kg/day orally at bedtime or divided twice daily; max dose 40 mg/day | ||
|warnings='''Allergy alert''': Do not use if you are allergic to famotidine or other acid reducers | |||
'''Do not use''' | |||
if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor. | |||
if you have kidney disease, except under the advice and supervision of a doctor | |||
with other acid reducers | |||
'''Ask a doctor before use if you have''' | |||
* Had heartburn over 3 months. This may be a sign of a more serious condition. | |||
* Heartburn with [[lightheadedness]], sweating, or [[dizziness]] | |||
* [[Chest pain]] or shoulder pain with [[shortness of breath]]; sweating; pain spreading to arms, neck or shoulders; or [[lightheadedness]] | |||
* | * Frequent chest pain | ||
* Frequent wheezing, particularly with heartburn | |||
* Unexplained weight loss | |||
* [[Nausea]] or [[vomiting]] | |||
* Stomach pain | |||
'''Stop use and ask a doctor if''' | |||
* Your heartburn continues or worsens | |||
* You need to take this product for more than 14 days | |||
'''If pregnant or breast-feeding''', | |||
* | * Ask a health professional before use. | ||
'''Keep out of reach of children'''. | |||
* In case of overdose, get medical help or contact a Poison Control Center right away. | |||
|clinicalTrials= | |clinicalTrials='''Common''' | ||
* Gastrointestinal: [[constipation]], [[diarrhea]] | |||
* Neurologic: [[dizziness]], [[headache]] | |||
'''Serious''' | |||
* Dermatologic: [[Stevens-Johnson syndrome]] (very rare ), toxic epidermal necrolysis (very rare ) | |||
* Gastrointestinal: necrotizing enterocolitis in fetus OR newborn | |||
* Immunologic: [[anaphylaxis]] (infrequent ), [[angioedema]] (infrequent ) | |||
* Musculoskeletal: [[rhabdomyolysis]] | |||
* Neurologic: [[seizure]] (rare ) | |||
* Respiratory: [[Interstitial pneumonia]] (infrequent ), [[nosocomial pneumonia]] | |||
|postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | ||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | ||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of | There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Famotidine in women who are pregnant. | ||
|useInLaborDelivery=There is no FDA guidance on use of | |useInLaborDelivery=There is no FDA guidance on use of Famotidine during labor and delivery. | ||
|useInNursing=There is no FDA guidance on the use of | |useInNursing=There is no FDA guidance on the use of Famotidine with respect to nursing mothers. | ||
|useInPed=There is no FDA guidance on the use of | |useInPed=There is no FDA guidance on the use of Famotidine with respect to pediatric patients. | ||
|useInGeri=There is no FDA guidance on the use of | |useInGeri=There is no FDA guidance on the use of Famotidine with respect to geriatric patients. | ||
|useInGender=There is no FDA guidance on the use of | |useInGender=There is no FDA guidance on the use of Famotidine with respect to specific gender populations. | ||
|useInRace=There is no FDA guidance on the use of | |useInRace=There is no FDA guidance on the use of Famotidine with respect to specific racial populations. | ||
|useInRenalImpair=There is no FDA guidance on the use of | |useInRenalImpair=There is no FDA guidance on the use of Famotidine in patients with renal impairment. | ||
|useInHepaticImpair=There is no FDA guidance on the use of | |useInHepaticImpair=There is no FDA guidance on the use of Famotidine in patients with hepatic impairment. | ||
|useInReproPotential=There is no FDA guidance on the use of | |useInReproPotential=There is no FDA guidance on the use of Famotidine in women of reproductive potentials and males. | ||
|useInImmunocomp=There is no FDA guidance one the use of | |useInImmunocomp=There is no FDA guidance one the use of Famotidine in patients who are immunocompromised. | ||
|administration=* Oral | |administration=* Oral | ||
|monitoring=There is limited information regarding <i>Monitoring</i> of Famotidine in the drug label. | |||
<!--IV Compatibility--> | <!--IV Compatibility--> | ||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of | |IVCompat=There is limited information regarding <i>IV Compatibility</i> of Famotidine in the drug label. | ||
|overdose=There is limited information regarding <i>Chronic Overdose</i> of Famotidine in the drug label. | |||
|drugBox=[[File:Famotidine structure.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
|overdose= | |PD=There is limited information regarding <i>Pharmacodynamics</i> of in the drug label. | ||
|PK=There is limited information regarding <i>Pharmacokinetics</i> of Famotidine in the drug label. | |||
|nonClinToxic=There is limited information regarding <i>Nonclinical Toxicology</i> of Famotidine in the drug label. | |||
|clinicalStudies=There is limited information regarding <i>Clinical Studies</i> of Famotidine in the drug label. | |||
|storage=* Store at 20°-25°C (68°-77°F) | |||
* Protect from moisture and light | |||
|fdaPatientInfo=There is limited information regarding <i>Patient Counseling Information</i> of Famotidine in the drug label. | |||
|alcohol=* Alcohol-Famotidine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
|brandNames=* 7 SELECT ACID CONTROLLER | |||
There is limited information regarding <i>Chronic Overdose</i> of | |||
|drugBox= | |||
|PD=There is limited information regarding <i>Pharmacodynamics</i> of | |||
|PK=There is limited information regarding <i>Pharmacokinetics</i> of | |||
|nonClinToxic=There is limited information regarding <i>Nonclinical Toxicology</i> of | |||
|clinicalStudies=There is limited information regarding <i>Clinical Studies</i> of | |||
| | |||
|fdaPatientInfo=There is limited information regarding <i>Patient Counseling Information</i> of | |||
|alcohol=* Alcohol- | |||
|brandNames=* | |||
|drugShortage= | |drugShortage= | ||
}} | }} | ||
| Line 309: | Line 170: | ||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
|fileName= | |fileName=Famotidine fig01.jpg | ||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
|fileName={{ | |fileName=Famotidine ingredients and appearance.png | ||
}} | |||
{{LabelImage | |||
|fileName=Famotidine fig.jpg | |||
}} | }} | ||
<!--Pill Image--> | <!--Pill Image--> | ||
| Line 325: | Line 189: | ||
<!--Category--> | <!--Category--> | ||
[[Category:Guanidines]] | |||
[[Category:Drug]] | [[Category:Drug]] | ||
Latest revision as of 14:21, 11 May 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
NOTE: Most over the counter (OTC) are not reviewed and approved by the FDA. However, they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Overview
Famotidine (oral) is a gastric acid secretion inhibitor that is FDA approved for the treatment of heartburn,gastric hypersecretion, gastric ulcer,gastroesophageal reflux, indigestion. Common adverse reactions include constipation ,diarrhea, dizziness , headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Duodenal ulcer
- Esophagitis - gastroesophageal reflux disease,
- Gastric hypersecretion
- Gastric ulcer
- Gastroesophageal reflux disease
- Indigestion
Dosing
- To relieve symptoms, swallow 1 tablet with a glass of water. Do not chew.
- To prevent symptoms, swallow 1 tablet with a glass of water at any time from 10 to 60 minutes before eating food or drinking beverages that cause heartburn
- Do not use more than 2 tablets in 24 hours
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of famotidine in adult patients.
Non–Guideline-Supported Use
Indications
- Esophagitis, Maintenance
- Gastritis
- Gastrointestinal hemorrhage
- Stress ulcer
- Urticaria
Dosing
- Duodenal ulcer disease: acute, 40 mg orally once daily at bedtime or 20 mg orally twice daily
- Duodenal ulcer disease: maintenance, 20 mg orally once daily at bedtime
- Esophagitis - Gastroesophageal reflux disease, Short term treatment: 20 to 40 mg orally twice daily for up to 12 weeks
- Gastric hypersecretion: 20 to 160 mg orally every 6 h.
- Gastric ulcer, Short term treatment: acute, 40 mg orally once daily at bedtime
- Gastroesophageal reflux disease, Short-term, symptom treatment: 20 mg orally twice daily for up to 6 wks
- Indigestion: 10 to 20 mg orally twice daily
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Indication
- Duodenal ulcer
- Esophagitis - gastroesophageal reflux disease,
- Gastric hypersecretion
- Gastric ulcer
- Gastroesophageal reflux disease
- Indigestion
Dosing
Children 12 years and over:
- To relieve symptoms, swallow 1 tablet with a glass of water. Do not chew.
- To prevent symptoms, swallow 1 tablet with a glass of water at any time from 10 to 60 minutes before eating food or drinking beverages that cause heartburn
- Do not use more than 2 tablets in 24 hours
Children under 12 years: ask a doctor
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Famotidine in pediatric patients.
Non–Guideline-Supported Use
Indications
- Stress ulcer; Prophylaxis
Dosage
- Duodenal ulcer disease: (over 1 y old) 0.5 mg/kg/day orally at bedtime or divided twice daily; max dose 40 mg/day
- Gastric ulcer, Short term treatment: (over 1 y old) 0.5 mg/kg/day orally at bedtime or divided twice daily; max dose 40 mg/day
Contraindications
There is limited information regarding Famotidine (oral) Contraindications in the drug label.
Warnings
Allergy alert: Do not use if you are allergic to famotidine or other acid reducers
Do not use
if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor. if you have kidney disease, except under the advice and supervision of a doctor with other acid reducers
Ask a doctor before use if you have
- Had heartburn over 3 months. This may be a sign of a more serious condition.
- Heartburn with lightheadedness, sweating, or dizziness
- Chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- Frequent chest pain
- Frequent wheezing, particularly with heartburn
- Unexplained weight loss
Stop use and ask a doctor if
- Your heartburn continues or worsens
- You need to take this product for more than 14 days
If pregnant or breast-feeding,
- Ask a health professional before use.
Keep out of reach of children.
- In case of overdose, get medical help or contact a Poison Control Center right away.
Adverse Reactions
Clinical Trials Experience
Common
- Gastrointestinal: constipation, diarrhea
- Neurologic: dizziness, headache
Serious
- Dermatologic: Stevens-Johnson syndrome (very rare ), toxic epidermal necrolysis (very rare )
- Gastrointestinal: necrotizing enterocolitis in fetus OR newborn
- Immunologic: anaphylaxis (infrequent ), angioedema (infrequent )
- Musculoskeletal: rhabdomyolysis
- Neurologic: seizure (rare )
- Respiratory: Interstitial pneumonia (infrequent ), nosocomial pneumonia
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Famotidine (oral) in the drug label.
Drug Interactions
There is limited information regarding Famotidine (oral) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Famotidine (oral) in women who are pregnant.
Pregnancy Category (AUS):
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Famotidine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Famotidine during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Famotidine with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Famotidine with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Famotidine with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Famotidine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Famotidine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Famotidine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Famotidine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Famotidine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Famotidine in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Famotidine in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Famotidine in the drug label.
Overdosage
There is limited information regarding Chronic Overdose of Famotidine in the drug label.
Pharmacology
Mechanism of Action
There is limited information regarding Famotidine (oral) Mechanism of Action in the drug label.
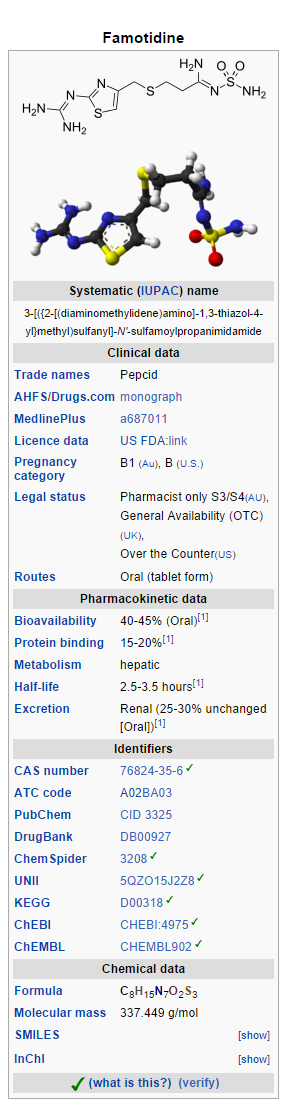
Structure
There is limited information regarding Famotidine (oral) Structure in the drug label.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Famotidine in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Famotidine in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Famotidine in the drug label.
How Supplied
There is limited information regarding Famotidine (oral) How Supplied in the drug label.
Storage
- Store at 20°-25°C (68°-77°F)
- Protect from moisture and light
Images
Drug Images
{{#ask: Page Name::Famotidine (oral) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Famotidine (oral) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Famotidine in the drug label.
Precautions with Alcohol
- Alcohol-Famotidine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- 7 SELECT ACID CONTROLLER
Look-Alike Drug Names
There is limited information regarding Famotidine (oral) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Famotidine (oral)
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Famotidine (oral) |Label Name=Famotidine fig01.jpg
}}
{{#subobject:
|Label Page=Famotidine (oral) |Label Name=Famotidine ingredients and appearance.png
}}
{{#subobject:
|Label Page=Famotidine (oral) |Label Name=Famotidine fig.jpg
}}