Exemestane
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Exemestane is {{{aOrAn}}} {{{drugClass}}} that is FDA approved for the {{{indicationType}}} of {{{indication}}}. Common adverse reactions include {{{adverseReactions}}}.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
There is limited information regarding Exemestane FDA-Labeled Indications and Dosage (Adult) in the drug label.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Exemestane in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Exemestane in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Exemestane FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Exemestane in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Exemestane in pediatric patients.
Contraindications
There is limited information regarding Exemestane Contraindications in the drug label.
Warnings
There is limited information regarding Exemestane Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Exemestane Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Exemestane Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Exemestane Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Exemestane in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Exemestane in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Exemestane during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Exemestane in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Exemestane in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Exemestane in geriatric settings.
Gender
There is no FDA guidance on the use of Exemestane with respect to specific gender populations.
Race
There is no FDA guidance on the use of Exemestane with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Exemestane in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Exemestane in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Exemestane in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Exemestane in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Exemestane Administration in the drug label.
Monitoring
There is limited information regarding Exemestane Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Exemestane and IV administrations.
Overdosage
There is limited information regarding Exemestane overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Exemestane Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Exemestane Mechanism of Action in the drug label.
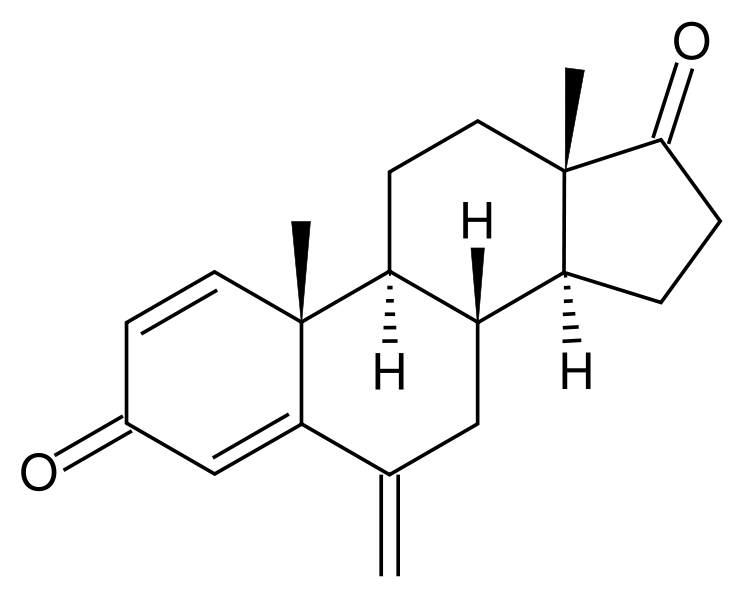
Structure
There is limited information regarding Exemestane Structure in the drug label.
Pharmacodynamics
There is limited information regarding Exemestane Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Exemestane Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Exemestane Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Exemestane Clinical Studies in the drug label.
How Supplied
There is limited information regarding Exemestane How Supplied in the drug label.
Storage
There is limited information regarding Exemestane Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Exemestane |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Exemestane |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Exemestane Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Exemestane interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Exemestane Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Exemestane Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | ~60% |
| Protein binding | 90% |
| Elimination half-life | 27 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C20H24O2 |
| Molar mass | 296.403 g/mol |
|
WikiDoc Resources for Exemestane |
|
Articles |
|---|
|
Most recent articles on Exemestane |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Exemestane at Clinical Trials.gov Clinical Trials on Exemestane at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Exemestane
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Exemestane Discussion groups on Exemestane Patient Handouts on Exemestane Directions to Hospitals Treating Exemestane Risk calculators and risk factors for Exemestane
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Exemestane |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Exemestane (trade name Aromasin) is an oral steroidal aromatase inhibitor (but also known uniquely as an aromatase inactivator) used in the adjuvant treatment of hormonally-responsive (also called hormone-receptor-positive, estrogen-responsive) breast cancer in postmenopausal women. An aim in the treatment of hormone-receptor-positive patients in preventing recurrence is to lower estrogen levels that this breast cancer thrives on.
The main source of estrogen is the ovaries in premenopausal women, while in post-menopausal women most of the body's estrogen is produced in the adrenal gland from the conversion of androgens into estrogen by the aromatase enzyme. Exemestane is an irreversible, steroidal aromatase inactivator, structurally related to the natural substrate androstenedione. It acts as a false substrate for the aromatase enzyme, and is processed to an intermediate that binds irreversibly to the active site of the enzyme causing its inactivation, an effect also known as "suicide inhibition." In other words, the Exemestane, by being structurally similar to the target of the enzymes, permanently binds to those enzymes, thereby preventing them from ever completing their task of converting androgens into estrogens.
The estrogen suppression rate for exemestane varies from 85% for estradiol (E2) to 95% for estrone (E1).
References
- Extensive information, including trial statistics from the National Institutes of Health
- Aromasin official website
- Profile of exemestane for use as an anti-estrogen by athletes and bodybuilders
- Coombes RC; et al. (2007). "Survival and safety of exemestane versus tamoxifen after 2–3 years' tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial". Lancet. 369 (9561): 559–70. doi:10.1016/S0140-6736(07)60200-1. PMID 17307102.
|access-date=requires|url=(help)
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Pages using citations with accessdate and no URL
- CS1 maint: Explicit use of et al.
- Aromatase inhibitors
- Endocrinology