Exemestane: Difference between revisions
m (Protected "Exemestane": Protecting pages from unwanted edits ([edit=sysop] (indefinite) [move=sysop] (indefinite))) |
m (Bot: Automated text replacement (-{{SIB}} + & -{{EJ}} + & -{{EH}} + & -{{Editor Join}} + & -{{Editor Help}} +)) |
||
| Line 1: | Line 1: | ||
| Line 35: | Line 35: | ||
{{Sex hormones}} | {{Sex hormones}} | ||
[[Category:Aromatase inhibitors]] | [[Category:Aromatase inhibitors]] | ||
[[Category:Endocrinology]] | [[Category:Endocrinology]] | ||
{{WikiDoc Help Menu}} | {{WikiDoc Help Menu}} | ||
{{WikiDoc Sources}} | {{WikiDoc Sources}} | ||
Revision as of 02:29, 9 August 2012
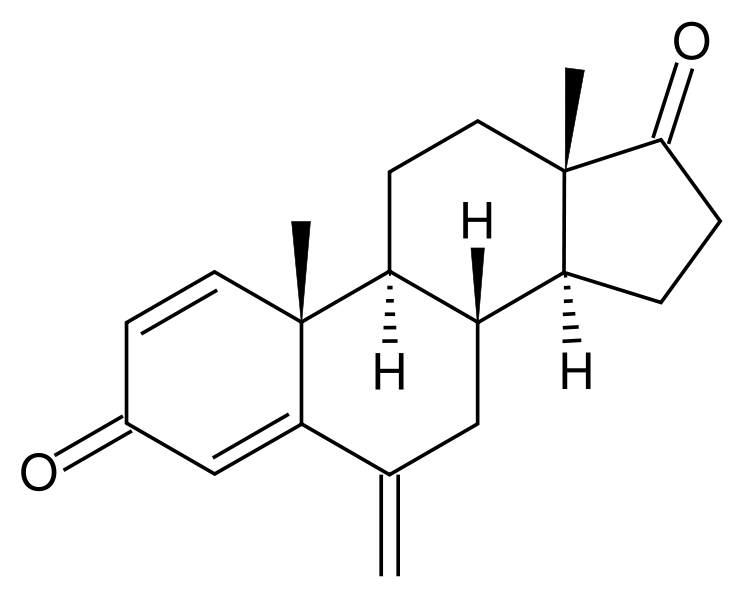 | |
| Clinical data | |
|---|---|
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | ~60% |
| Protein binding | 90% |
| Elimination half-life | 27 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C20H24O2 |
| Molar mass | 296.403 g/mol |
|
WikiDoc Resources for Exemestane |
|
Articles |
|---|
|
Most recent articles on Exemestane |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Exemestane at Clinical Trials.gov Clinical Trials on Exemestane at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Exemestane
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Exemestane Discussion groups on Exemestane Patient Handouts on Exemestane Directions to Hospitals Treating Exemestane Risk calculators and risk factors for Exemestane
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Exemestane |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Exemestane (trade name Aromasin) is an oral steroidal aromatase inhibitor (but also known uniquely as an aromatase inactivator) used in the adjuvant treatment of hormonally-responsive (also called hormone-receptor-positive, estrogen-responsive) breast cancer in postmenopausal women. An aim in the treatment of hormone-receptor-positive patients in preventing recurrence is to lower estrogen levels that this breast cancer thrives on.
The main source of estrogen is the ovaries in premenopausal women, while in post-menopausal women most of the body's estrogen is produced in the adrenal gland from the conversion of androgens into estrogen by the aromatase enzyme. Exemestane is an irreversible, steroidal aromatase inactivator, structurally related to the natural substrate androstenedione. It acts as a false substrate for the aromatase enzyme, and is processed to an intermediate that binds irreversibly to the active site of the enzyme causing its inactivation, an effect also known as "suicide inhibition." In other words, the Exemestane, by being structurally similar to the target of the enzymes, permanently binds to those enzymes, thereby preventing them from ever completing their task of converting androgens into estrogens.
The estrogen suppression rate for exemestane varies from 85% for estradiol (E2) to 95% for estrone (E1).
References
- Extensive information, including trial statistics from the National Institutes of Health
- Aromasin official website
- Profile of exemestane for use as an anti-estrogen by athletes and bodybuilders
- Coombes RC; et al. (2007). "Survival and safety of exemestane versus tamoxifen after 2–3 years' tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial". Lancet. 369 (9561): 559–70. doi:10.1016/S0140-6736(07)60200-1. PMID 17307102.
|access-date=requires|url=(help)
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Pages using citations with accessdate and no URL
- CS1 maint: Explicit use of et al.
- Aromatase inhibitors
- Endocrinology