Everolimus
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Everolimus is an antineoplastic agent that is FDA approved for the treatment of advanced hormone receptor-positive, HER2-negative breast cancer (advanced HR+ BC) in combination with exemestane after failure of treatment with letrozole or anastrozole, progressive neuroendocrine tumors of pancreatic origin (PNET) that are unresectable, locally advanced or metastatic, advanced renal cell carcinoma (RCC) after failure of treatment with sunitinib or sorafenib, renal angiomyolipoma and tuberous sclerosis complex (TSC), not requiring immediate surgery and tuberous sclerosis complex (TSC) who have subependymal giant cell astrocytoma (SEGA). Common adverse reactions include stomatitis, infections, rash, fatigue, diarrhea, edema, abdominal pain, nausea, fever, asthenia, cough, headache and decreased appetite.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Advanced Hormone Receptor-Positive, HER2-Negative Breast Cancer (Advanced HR+ BC)
- AFINITOR® is indicated for the treatment of postmenopausal women with advanced hormone receptor-positive, HER2-negative breast cancer (advanced HR+ BC) in combination with exemestane, after failure of treatment with letrozole or anastrozole.
Advanced Neuroendocrine Tumors of Pancreatic Origin (PNET)
- AFINITOR® is indicated for the treatment of adult patients with progressive neuroendocrine tumors of pancreatic origin (PNET) with unresectable, locally advanced or metastatic disease.
- AFINITOR® is not indicated for the treatment of patients with functional carcinoid tumors.
Advanced Renal Cell Carcinoma (RCC)
- AFINITOR® is indicated for the treatment of adult patients with advanced renal cell carcinoma (RCC) after failure of treatment with sunitinib or sorafenib.
Renal Angiomyolipoma with Tuberous Sclerosis Complex (TSC)
- AFINITOR® is indicated for the treatment of adult patients with renal angiomyolipoma and tuberous sclerosis complex (TSC), not requiring immediate surgery.
- The effectiveness of AFINITOR in the treatment of renal angiomyolipoma is based on an analysis of durable objective responses in patients treated for a median of 8.3 months. Further follow-up of patients is required to determine long-term outcomes.
Subependymal Giant Cell Astrocytoma (SEGA) with Tuberous Sclerosis Complex (TSC)
- AFINITOR® Tablets and AFINITOR® DISPERZ are indicated in pediatric and adult patients with tuberous sclerosis complex (TSC) for the treatment of subependymal giant cell astrocytoma (SEGA) that requires therapeutic intervention but cannot be curatively resected.
- The effectiveness of AFINITOR Tablets and AFINITOR DISPERZ is based on demonstration of durable objective response, as evidenced by reduction in SEGA tumor volume. Improvement in disease-related symptoms and overall survival in patients with SEGA and TSC has not been demonstrated.
DOSAGE AND ADMINISTRATION
- AFINITOR is available in two dosage forms: tablets (AFINITOR Tablets) and tablets for oral suspension (AFINITOR DISPERZ).
- AFINITOR Tablets may be used for all approved indications.
- AFINITOR DISPERZ is approved for the treatment of patients with subependymal giant cell astrocytoma (SEGA) and tuberous sclerosis complex (TSC).
Recommended Dose in Advanced Hormone Receptor-Positive, HER2-Negative Breast Cancer, Advanced PNET, Advanced RCC, and Renal Angiomyolipoma with TSC
- The recommended dose of AFINITOR Tablets is 10 mg, to be taken once daily at the same time every day. Administer either consistently with food or consistently without food. AFINITOR Tablets should be swallowed whole with a glass of water. Do not break or crush tablets.
- Continue treatment until disease progression or unacceptable toxicity occurs.
Dose Modifications in Advanced Hormone Receptor-Positive, HER2-Negative Breast Cancer, Advanced PNET, Advanced RCC, and Renal Angiomyolipoma with TSC
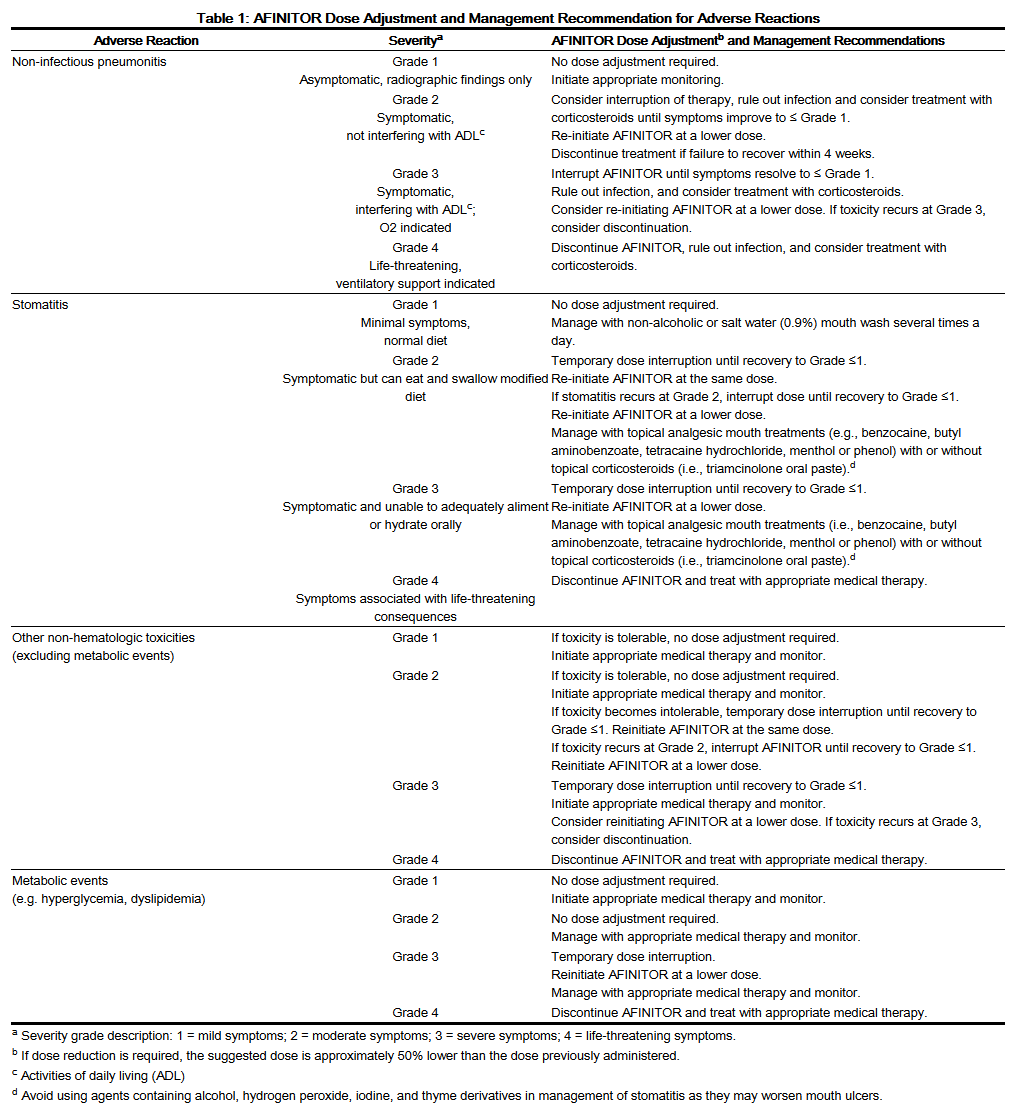
- Adverse Reactions
- Management of severe or intolerable adverse reactions may require temporary dose interruption (with or without a dose reduction of AFINITOR therapy) or discontinuation. If dose reduction is required, the suggested dose is approximately 50% lower than the daily dose previously administered.
- Table 1 summarizes recommendations for dose reduction, interruption or discontinuation of AFINITOR in the management of adverse reactions. General management recommendations are also provided as applicable. Clinical judgment of the treating physician should guide the management plan of each patient based on individual benefit/risk assessment.
- Hepatic Impairment
- Hepatic impairment will increase the exposure to everolimus. Dose adjustments are recommended:
- Mild hepatic impairment (Child-Pugh class A) – The recommended dose is 7.5 mg daily; the dose may be decreased to 5 mg if not well tolerated.
- Moderate hepatic impairment (Child-Pugh class B) – The recommended dose is 5 mg daily; the dose may be decreased to 2.5 mg if not well tolerated.
- Severe hepatic impairment (Child-Pugh class C) – If the desired benefit outweighs the risk, a dose of 2.5 mg daily may be used but must not be exceeded.
- Hepatic impairment will increase the exposure to everolimus. Dose adjustments are recommended:
- Dose adjustments should be made if a patient’s hepatic (Child-Pugh) status changes during treatment.
- CYP3A4/P-glycoprotein (PgP) Inhibitors
- Avoid the use of strong CYP3A4/PgP inhibitors (e.g., ketoconazole, itraconazole, clarithromycin, atazanavir, nefazodone, saquinavir, telithromycin, ritonavir, indinavir, nelfinavir, voriconazole).
- Use caution when co-administered with moderate CYP3A4/PgP inhibitors (e.g., amprenavir, fosamprenavir, aprepitant, erythromycin, fluconazole, verapamil, diltiazem). If patients require co-administration of a moderate CYP3A4/PgP inhibitor, reduce the AFINITOR dose to 2.5 mg daily. The reduced dose of AFINITOR is predicted to adjust the area under the curve (AUC) to the range observed without inhibitors. An AFINITOR dose increase from 2.5 mg to 5 mg may be considered based on patient tolerance. If the moderate inhibitor is discontinued, a washout period of approximately 2 to 3 days should be allowed before the AFINITOR dose is increased. If the moderate inhibitor is discontinued, the AFINITOR dose should be returned to the dose used prior to initiation of the moderate CYP3A4/PgP inhibitor.
- Grapefruit, grapefruit juice, and other foods that are known to inhibit cytochrome P450 and PgP activity may increase everolimus exposures and should be avoided during treatment.
- Strong CYP3A4/PgP Inducers
- Avoid the use of concomitant strong CYP3A4/PgP inducers (e.g., phenytoin, carbamazepine, rifampin, rifabutin, rifapentine, phenobarbital). If patients require co-administration of a strong CYP3A4/PgP inducer, consider doubling the daily dose of AFINITOR using increments of 5 mg or less. This dose of AFINITOR is predicted, based on pharmacokinetic data, to adjust the AUC to the range observed without inducers. However, there are no clinical data with this dose adjustment in patients receiving strong CYP3A4/PgP inducers. If the strong inducer is discontinued, consider a washout period of 3 to 5 days, before the AFINITOR dose is returned to the dose used prior to initiation of the strong CYP3A4/PgP inducer.
- St. John’s Wort (Hypericum perforatum) may decrease everolimus exposure unpredictably and should be avoided.
Recommended Dose in SEGA with TSC
- The recommended starting dose is 4.5 mg/m2, once daily. The recommended starting dose for patients with severe hepatic impairment (Child-Pugh class C) or requiring moderate CYP3A4/PgP inhibitors is 2.5 mg/m2, once daily. The recommended starting dose for patients requiring a concomitant strong CYP3A4 inducer is 9 mg/m2, once daily. Round dose to the nearest strength of either AFINITOR Tablets or AFINITOR DISPERZ.
- Do not combine AFINITOR Tablets and AFINITOR DISPERZ to achieve the desired total dose.
- Use therapeutic drug monitoring to guide subsequent dosing. Adjust dose at 2 week intervals as needed to achieve and maintain trough concentrations of 5 to 15 ng/mL.
- Continue treatment until disease progression or unacceptable toxicity occurs. The optimal duration of therapy is unknown.
Therapeutic Drug Monitoring in SEGA with TSC
- Monitor everolimus whole blood trough levels routinely in all patients. When possible, use the same assay and laboratory for therapeutic drug monitoring throughout treatment.
- Assess trough concentrations approximately 2 weeks after initiation of treatment, a change in dose, a change in co-administration of CYP3A4/PgP inducers and/or inhibitors, a change in hepatic function, or a change in dosage form between AFINITOR Tablets and AFINITOR DISPERZ. Once a stable dose is attained, monitor trough concentrations every 3 to 6 months in patients with changing body surface area or every 6 to 12 months in patients with stable body surface area for the duration of treatment.
- Titrate the dose to attain trough concentrations of 5 to 15 ng/mL.
- For trough concentrations less than 5 ng/mL, increase the daily dose by 2.5 mg (in patients taking AFINITOR Tablets) or 2 mg (in patients taking AFINITOR DISPERZ).
- For trough concentrations greater than 15 ng/mL, reduce the daily dose by 2.5 mg (in patients taking AFINITOR Tablets) or 2 mg (in patients taking AFINITOR DISPERZ).
- If dose reduction is required for patients receiving the lowest available strength, administer every other day.
Dose Modifications in SEGA with TSC
- Adverse Reactions
- Temporarily interrupt or permanently discontinue AFINITOR Tablets or AFINITOR DISPERZ for severe or intolerable adverse reactions. If dose reduction is required when reinitiating therapy, reduce the dose by approximately 50%. If dose reduction is required for patients receiving the lowest available strength, administer every other day.
- Hepatic Impairment
- Reduce the starting dose of AFINITOR Tablets or AFINITOR DISPERZ by approximately 50% in patients with SEGA who have severe hepatic impairment (Child-Pugh class C). Adjustment to the starting dose for patients with SEGA who have mild (Child-Pugh class A) or moderate (Child-Pugh class B) hepatic impairment may not be needed. Subsequent dosing should be based on therapeutic drug monitoring.
- Assess everolimus trough concentrations approximately 2 weeks after commencing treatment, a change in dose, or any change in hepatic function.
- CYP3A4/P-glycoprotein (PgP) Inhibitors
- Avoid the use of concomitant strong CYP3A4/PgP inhibitors (e.g., ketoconazole, itraconazole, clarithromycin, atazanavir, nefazodone, saquinavir, telithromycin, ritonavir, indinavir, nelfinavir, voriconazole) in patients receiving AFINITOR Tablets or AFINITOR DISPERZ.
- For patients who require treatment with moderate CYP3A4/PgP inhibitors (e.g., amprenavir, fosamprenavir, aprepitant, erythromycin, fluconazole, verapamil, diltiazem):
- Reduce the AFINITOR Tablets or AFINITOR DISPERZ dose by approximately 50%. Administer every other day if dose reduction is required for patients receiving the lowest available strength and maintain trough concentrations of 5 to 15 ng/mL.
- Assess everolimus trough concentrations approximately 2 weeks after dose reduction.
- Resume the dose that was used prior to initiating the CYP3A4/PgP inhibitor 2 to 3 days after discontinuation of a moderate inhibitor. Assess the everolimus trough concentration approximately 2 weeks later.
- Do not ingest foods or nutritional supplements (e.g., grapefruit, grapefruit juice) that are known to inhibit cytochrome P450 or PgP activity.
- Strong CYP3A4/PgP Inducers
- Avoid the use of concomitant strong CYP3A4/PgP inducers (e.g., phenytoin, carbamazepine, rifampin, rifabutin, rifapentine, phenobarbital) if alternative therapy is available. For patients who require treatment with a strong CYP3A4/PgP inducer:
- Double the dose of AFINITOR Tablets or AFINITOR DISPERZ and assess tolerability.
- Assess the everolimus trough concentration 2 weeks after doubling the dose and adjust the dose if necessary to maintain a trough concentration of 5 to 15 ng/mL.
- Return the AFINITOR Tablets or AFINITOR DISPERZ dose to that used prior to initiating the strong CYP3A4/PgP inducer if the strong inducer is discontinued, and assess the everolimus trough concentrations approximately 2 weeks later.
- Avoid the use of concomitant strong CYP3A4/PgP inducers (e.g., phenytoin, carbamazepine, rifampin, rifabutin, rifapentine, phenobarbital) if alternative therapy is available. For patients who require treatment with a strong CYP3A4/PgP inducer:
- Do not ingest foods or nutritional supplements (e.g., St. John’s Wort (Hypericum perforatum)) that are known to induce cytochrome P450 activity.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Everolimus in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Everolimus in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Subependymal Giant Cell Astrocytoma (SEGA) with Tuberous Sclerosis Complex (TSC)
- Dosing Information
- The recommended starting dose is 4.5 mg/m2, once daily. The recommended starting dose for patients with severe hepatic impairment (Child-Pugh class C) or requiring moderate CYP3A4/PgP inhibitors is 2.5 mg/m2, once daily. The recommended starting dose for patients requiring a concomitant strong CYP3A4 inducer is 9 mg/m2, once daily. Round dose to the nearest strength of either AFINITOR Tablets or AFINITOR DISPERZ.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Everolimus in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Everolimus in pediatric patients.
Contraindications
- AFINITOR is contraindicated in patients with hypersensitivity to the active substance, to other rapamycin derivatives, or to any of the excipients. Hypersensitivity reactions manifested by symptoms including, but not limited to, anaphylaxis, dyspnea, flushing, chest pain, or angioedema (e.g., swelling of the airways or tongue, with or without respiratory impairment) have been observed with everolimus and other rapamycin derivatives.
Warnings
Precautions
- Non-infectious Pneumonitis
- Non-infectious pneumonitis is a class effect of rapamycin derivatives, including AFINITOR. Non-infectious pneumonitis was reported in up to 19% of patients treated with AFINITOR in clinical trials. The incidence of Common Terminology Criteria (CTC) Grade 3 and 4 non-infectious pneumonitis was up to 4.0% and up to 0.2%, respectively. Fatal outcomes have been observed.
- Consider a diagnosis of non-infectious pneumonitis in patients presenting with non-specific respiratory signs and symptoms such as hypoxia, pleural effusion, cough, or dyspnea, and in whom infectious, neoplastic, and other causes have been excluded by means of appropriate investigations. Opportunistic infections such as pneumocystis jiroveci pneumonia (PJP) should be considered in the differential diagnosis. Advise patients to report promptly any new or worsening respiratory symptoms.
- Patients who develop radiological changes suggestive of non-infectious pneumonitis and have few or no symptoms may continue AFINITOR therapy without dose alteration. Imaging appears to overestimate the incidence of clinical pneumonitis.
- If symptoms are moderate, consider interrupting therapy until symptoms improve. The use of corticosteroids may be indicated. AFINITOR may be reintroduced at a daily dose approximately 50% lower than the dose previously administered.
- For cases of Grade 3 non-infectious pneumonitis interrupt AFINITOR until resolution to less than or equal to Grade 1. AFINITOR may be re-introduced at a daily dose approximately 50% lower than the dose previously administered depending on the individual clinical circumstances. If toxicity recurs at Grade 3, consider discontinuation of AFINITOR. For cases of Grade 4 non-infectious pneumonitis, discontinue AFINITOR. Corticosteroids may be indicated until clinical symptoms resolve. For patients who require use of corticosteroids for treatment of non-infectious pneumonitis, prophylaxis for PJP may be considered. The development of pneumonitis has been reported even at a reduced dose.
- Infections
- AFINITOR has immunosuppressive properties and may predispose patients to bacterial, fungal, viral, or protozoal infections, including infections with opportunistic pathogens. Localized and systemic infections, including pneumonia, mycobacterial infections, other bacterial infections, invasive fungal infections, such as aspergillosis, candidiasis, or pneumocystis jiroveci pneumonia (PJP) and viral infections including reactivation of hepatitis B virus have occurred in patients taking AFINITOR. Some of these infections have been severe (e.g., leading to sepsis, respiratory or hepatic failure) or fatal. Physicians and patients should be aware of the increased risk of infection with AFINITOR. Complete treatment of pre-existing invasive fungal infections prior to starting treatment with AFINITOR. While taking AFINITOR, be vigilant for signs and symptoms of infection; if a diagnosis of an infection is made, institute appropriate treatment promptly and consider interruption or discontinuation of AFINITOR. If a diagnosis of invasive systemic fungal infection is made, discontinue AFINITOR and treat with appropriate antifungal therapy.
- Pneumocystis jiroveci pneumonia, some with a fatal outcome, has been reported in patients who received everolimus. This may be associated with concomitant use of corticosteroids or other immunosuppressive agents. Prophylaxis for PJP should be considered when concomitant use of corticosteroids or other immunosuppressive agents are required.
- Oral Ulceration
- Mouth ulcers, stomatitis, and oral mucositis have occurred in patients treated with AFINITOR at an incidence ranging from 44%-78% across the clinical trial experience. Grade 3 or 4 stomatitis was reported in 4%-9% of patients. In such cases, topical treatments are recommended, but alcohol-, hydrogen peroxide-, iodine-, or thyme- containing mouthwashes should be avoided as they may exacerbate the condition. Antifungal agents should not be used unless fungal infection has been diagnosed.
- Renal Failure
- Cases of renal failure (including acute renal failure), some with a fatal outcome, have been observed in patients treated with AFINITOR.
- Impaired Wound Healing
- Everolimus delays wound healing and increases the occurrence of wound-related complications like wound dehiscence, wound infection, incisional hernia, lymphocele, and seroma. These wound-related complications may require surgical intervention. Exercise caution with the use of AFINITOR in the peri-surgical period.
- Geriatric Patients
- In the randomized advanced hormone receptor-positive, HER2-negative breast cancer study, the incidence of deaths due to any cause within 28 days of the last AFINITOR dose was 6% in patients ≥ 65 years of age compared to 2% in patients < 65 years of age. Adverse reactions leading to permanent treatment discontinuation occurred in 33% of patients ≥ 65 years of age compared to 17% in patients < 65 years of age. Careful monitoring and appropriate dose adjustments for adverse reactions are recommended.
- Laboratory Tests and Monitoring
- Renal Function
- Elevations of serum creatinine and proteinuria have been reported in patients taking AFINITOR. Monitoring of renal function, including measurement of blood urea nitrogen (BUN), urinary protein, or serum creatinine, is recommended prior to the start of AFINITOR therapy and periodically thereafter. Renal function of patients should be monitored particularly where patients have additional risk factors that may further impair renal function.
- Blood Glucose and Lipids
- Hyperglycemia, hyperlipidemia, and hypertriglyceridemia have been reported in patients taking AFINITOR. Monitoring of fasting serum glucose and lipid profile is recommended prior to the start of AFINITOR therapy and periodically thereafter as well as management with appropriate medical therapy. More frequent monitoring is recommended when AFINITOR is co-administered with other drugs that may induce hyperglycemia. When possible, optimal glucose and lipid control should be achieved before starting a patient on AFINITOR.
- Hematologic Parameters
- Decreased hemoglobin, lymphocytes, neutrophils, and platelets have been reported in patients taking AFINITOR. Monitoring of complete blood count is recommended prior to the start of AFINITOR therapy and periodically thereafter.
- Drug-drug Interactions
- Due to significant increases in exposure of everolimus, co-administration with strong CYP3A4/PgP inhibitors should be avoided.
- A reduction of the AFINITOR dose is recommended when co-administered with a moderate CYP3A4/PgP inhibitor.
- An increase in the AFINITOR dose is recommended when co-administered with a strong CYP3A4/PgP inducer.
- Hepatic Impairment
- Exposure to everolimus was increased in patients with hepatic impairment.
- For advanced HR+ BC, advanced PNET, advanced RCC, and renal angiomyolipoma with TSC patients with severe hepatic impairment (Child-Pugh class C), AFINITOR may be used at a reduced dose if the desired benefit outweighs the risk. For patients with mild (Child-Pugh class A) or moderate (Child-Pugh class B) hepatic impairment, a dose reduction is recommended.
- For patients with SEGA and mild or moderate hepatic impairment, adjust the dose of AFINITOR Tablets or AFINITOR DISPERZ based on therapeutic drug monitoring. For patients with SEGA and severe hepatic impairment, reduce the starting dose of AFINITOR Tablets or AFINITOR DISPERZ by approximately 50% and adjust subsequent doses based on therapeutic drug monitoring.
- Vaccinations
- During AFINITOR treatment, avoid the use of live vaccines and avoid close contact with individuals who have received live vaccines (e.g., intranasal influenza, measles, mumps, rubella, oral polio, BCG, yellow fever, varicella, and TY21a typhoid vaccines).
- For pediatric patients with SEGA that do not require immediate treatment, complete the recommended childhood series of live virus vaccinations according to American Council on Immunization Practices (ACIP) guidelines prior to the start of therapy. An accelerated vaccination schedule may be appropriate.
- Embryo-fetal Toxicity
- Based on the mechanism of action, AFINITOR can cause fetal harm. Everolimus caused embryo-fetal toxicities in animals at maternal exposures that were lower than human exposures. If this drug is used during pregnancy or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus.
- Advise female patients of reproductive potential to avoid becoming pregnant and to use highly effective contraception while using AFINITOR and for up to 8 weeks after ending treatment.
Adverse Reactions
Clinical Trials Experience
- The efficacy and safety of AFINITOR (10 mg/day) plus exemestane (25 mg/day) (n=485) versus placebo plus exemestane (25 mg/day) (n=239) was evaluated in a randomized, controlled trial in patients with advanced or metastatic hormone receptor-positive, HER2-negative breast cancer. The median age of patients was 61 years (range 28-93 years), and 75% were Caucasian. Safety results are based on a median follow-up of approximately 13 months.
- The most common adverse reactions (incidence ≥ 30%) were stomatitis, infections, rash, fatigue, diarrhea, and decreased appetite. The most common Grade 3/4 adverse reactions (incidence ≥ 2%) were stomatitis, infections, hyperglycemia, fatigue, dyspnea, pneumonitis, and diarrhea. The most common laboratory abnormalities (incidence ≥ 50%) were hypercholesterolemia, hyperglycemia, increased aspartate transaminase (AST), anemia, leukopenia, thrombocytopenia, lymphopenia, increased alanine transaminase (ALT), and hypertriglyceridemia. The most common Grade 3/4 laboratory abnormalities (incidence ≥ 3%) were lymphopenia, hyperglycemia, anemia, decreased potassium, increased AST, increased ALT, and thrombocytopenia.
- Fatal adverse reactions occurred more frequently in patients who received AFINITOR plus exemestane (2%) compared to patients on the placebo plus exemestane arm (0.4%). The rates of treatment-emergent adverse events resulting in permanent discontinuation were 24% and 5% for the AFINITOR plus exemestane and placebo plus exemestane treatment groups, respectively. Dose adjustments (interruptions or reductions) were more frequent among patients in the AFINITOR plus exemestane arm than in the placebo plus exemestane arm (63% versus 14%).
- Table 2 compares the incidence of treatment-emergent adverse reactions reported with an incidence of ≥10% for patients receiving AFINITOR 10 mg daily versus placebo.
- Key observed laboratory abnormalities are presented in Table 3.
Clinical Study Experience in Advanced Pancreatic Neuroendocrine Tumors
- In a randomized, controlled trial of AFINITOR (n=204) versus placebo (n=203) in patients with advanced PNET the median age of patients was 58 years (range 20-87), 79% were Caucasian, and 55% were male. Patients on the placebo arm could cross over to open-label AFINITOR upon disease progression.
- The most common adverse reactions (incidence ≥ 30%) were stomatitis, rash, diarrhea, fatigue, edema, abdominal pain, nausea, fever, and headache. The most common Grade 3-4 adverse reactions (incidence ≥ 5%) were stomatitis and diarrhea. The most common laboratory abnormalities (incidence ≥ 50%) were decreased hemoglobin, hyperglycemia, alkaline phosphatase increased, hypercholesterolemia, bicarbonate decreased, and increased aspartate transaminase (AST). The most common Grade 3-4 laboratory abnormalities (incidence ≥ 3%) were hyperglycemia, lymphopenia, decreased hemoglobin, hypophosphatemia, increased alkaline phosphatase, neutropenia, increased aspartate transaminase (AST), potassium decreased, and thrombocytopenia. Deaths during double-blind treatment where an adverse event was the primary cause occurred in seven patients on AFINITOR and one patient on placebo. Causes of death on the AFINITOR arm included one case of each of the following: acute renal failure, acute respiratory distress, cardiac arrest, death (cause unknown), hepatic failure, pneumonia, and sepsis. There was one death due to pulmonary embolism on the placebo arm. After cross-over to open-label AFINITOR, there were three additional deaths, one due to hypoglycemia and cardiac arrest in a patient with insulinoma, one due to myocardial infarction with congestive heart failure, and the other due to sudden death. The rates of treatment-emergent adverse events resulting in permanent discontinuation were 20% and 6% for the AFINITOR and placebo treatment groups, respectively. Dose delay or reduction was necessary in 61% of everolimus patients and 29% of placebo patients. Grade 3-4 renal failure occurred in six patients in the everolimus arm and three patients in the placebo arm. Thrombotic events included five patients with pulmonary embolus in the everolimus arm and one in the placebo arm as well as three patients with thrombosis in the everolimus arm and two in the placebo arm.
- Table 4 compares the incidence of treatment-emergent adverse reactions reported with an incidence of ≥ 10% for patients receiving AFINITOR 10 mg daily versus placebo.
- In female patients aged 18 to 55 years, irregular menstruation occurred in 5 of 46 (11%) AFINITOR-treated females and none of the 33 females in the placebo group.
- Key observed laboratory abnormalities are presented in Table 5.
Clinical Study Experience in Advanced Renal Cell Carcinoma
- The data described below reflect exposure to AFINITOR (n=274) and placebo (n=137) in a randomized, controlled trial in patients with metastatic renal cell carcinoma who received prior treatment with sunitinib and/or sorafenib. The median age of patients was 61 years (range 27-85), 88% were Caucasian, and 78% were male. The median duration of blinded study treatment was 141 days (range 19-451 days) for patients receiving AFINITOR and 60 days (range 21-295 days) for those receiving placebo.
- The most common adverse reactions (incidence ≥ 30%) were stomatitis, infections, asthenia, fatigue, cough, and diarrhea. The most common Grade 3-4 adverse reactions (incidence ≥ 3%) were infections, dyspnea, fatigue, stomatitis, dehydration, pneumonitis, abdominal pain, and asthenia. The most common laboratory abnormalities (incidence ≥ 50%) were anemia, hypercholesterolemia, hypertriglyceridemia, hyperglycemia, lymphopenia, and increased creatinine. The most common Grade 3-4 laboratory abnormalities (incidence ≥ 3%) were lymphopenia, hyperglycemia, anemia, hypophosphatemia, and hypercholesterolemia. Deaths due to acute respiratory failure (0.7%), infection (0.7%), and acute renal failure (0.4%) were observed on the AFINITOR arm but none on the placebo arm. The rates of treatment-emergent adverse events (irrespective of causality) resulting in permanent discontinuation were 14% and 3% for the AFINITOR and placebo treatment groups, respectively. The most common adverse reactions (irrespective of causality) leading to treatment discontinuation were pneumonitis and dyspnea. Infections, stomatitis, and pneumonitis were the most common reasons for treatment delay or dose reduction. The most common medical interventions required during AFINITOR treatment were for infections, anemia, and stomatitis.
- Table 6 compares the incidence of treatment-emergent adverse reactions reported with an incidence of ≥ 10% for patients receiving AFINITOR 10 mg daily versus placebo. Within each MedDRA system organ class, the adverse reactions are presented in order of decreasing frequency.
- Other notable adverse reactions occurring more frequently with AFINITOR than with placebo, but with an incidence of < 10% include:
- Gastrointestinal disorders: Abdominal pain (9%), dry mouth (8%), hemorrhoids (5%), dysphagia (4%)
- General disorders and administration site conditions: Weight decreased (9%), chest pain (5%), chills (4%), impaired wound healing (< 1%)
- Respiratory, thoracic and mediastinal disorders: Pleural effusion (7%), pharyngolaryngeal pain (4%), rhinorrhea (3%)
- Skin and subcutaneous tissue disorders: Hand-foot syndrome (reported as palmar-plantar erythrodysesthesia syndrome) (5%), nail disorder (5%), erythema (4%), onychoclasis (4%), skin lesion (4%), acneiform dermatitis (3%)
- Metabolism and nutrition disorders: Exacerbation of pre-existing diabetes mellitus (2%), new onset of diabetes mellitus (< 1%)
- Psychiatric disorders: Insomnia (9%)
- Nervous system disorders: Dizziness (7%), paresthesia (5%)
- Eye disorders: Eyelid edema (4%), conjunctivitis (2%)
- Vascular disorders: Hypertension (4%), deep vein thrombosis (< 1%)
- Renal and urinary disorders: Renal failure (3%)
- Cardiac disorders: Tachycardia (3%), congestive cardiac failure (1%)
- Musculoskeletal and connective tissue disorders: Jaw pain (3%)
- Hematologic disorders: Hemorrhage (3%)
- Key laboratory abnormalities are presented in Table 7.
Clinical Study Experience in Renal Angiomyolipoma with Tuberous Sclerosis Complex
- The data described below are based on a randomized (2:1), double-blind, placebo-controlled trial of AFINITOR in 118 patients with renal angiomyolipoma as a feature of TSC (n=113) or sporadic lymphangioleiomyomatosis (n=5). The median age of patients was 31 years (range 18 to 61 years), 89% were Caucasian, and 34% were male. The median duration of blinded study treatment was 48 weeks (range 2 to 115 weeks) for patients receiving AFINITOR and 45 weeks (range 9 to 115 weeks) for those receiving placebo.
- The most common adverse reaction reported for AFINITOR (incidence ≥ 30%) was stomatitis. The most common Grade 3-4 adverse reactions (incidence ≥ 2%) were stomatitis and amenorrhea. The most common laboratory abnormalities (incidence ≥ 50%) were hypercholesterolemia, hypertriglyceridemia, and anemia. The most common Grade 3-4 laboratory abnormality (incidence ≥ 3%) was hypophosphatemia.
- The rate of adverse reactions resulting in permanent discontinuation was 3.8% in the AFINITOR-treated patients. Adverse reactions leading to permanent discontinuation in the AFINITOR arm were hypersensitivity/angioedema/bronchospasm, convulsion, and hypophosphatemia. Dose adjustments (interruptions or reductions) due to adverse reactions occurred in 52% of AFINITOR-treated patients. The most common adverse reaction leading to AFINITOR dose adjustment was stomatitis.
- Table 8 compares the incidence of adverse reactions reported with an incidence of ≥ 10% for patients receiving AFINITOR and occurring more frequently with AFINITOR than with placebo. Laboratory abnormalities are described separately in Table 9.
- Amenorrhea occurred in 15% of AFINITOR-treated females (8 of 52) and 4% (1 of 26) of females in the placebo group. Other adverse reactions involving the female reproductive system were menorrhagia (10%), menstrual irregularities (10%), and vaginal hemorrhage (8%).
- The following additional adverse reactions occurred in less than 10% of Afinitor-treated patients: epistaxis (9%), decreased appetite (6%), otitis media (6%), depression (5%), abnormal taste (5%), increased blood luteinizing hormone (LH) levels (4%), increased blood follicle stimulating hormone (FSH) levels (3%), hypersensitivity (3%), and pneumonitis (1%).
Clinical Study Experience in Subependymal Giant Cell Astrocytoma with Tuberous Sclerosis Complex
- The data described below are based on a randomized (2:1), double-blind, placebo-controlled trial (Study 1) of AFINITOR in 117 patients with subependymal giant cell astrocytoma (SEGA) and tuberous sclerosis complex (TSC). The median age of patients was 9.5 years (range 0.8 to 26 years), 93% were Caucasian, and 57% were male. The median duration of blinded study treatment was 52 weeks (range 24 to 89 weeks) for patients receiving AFINITOR and 47 weeks (range 14 to 88 weeks) for those receiving placebo.
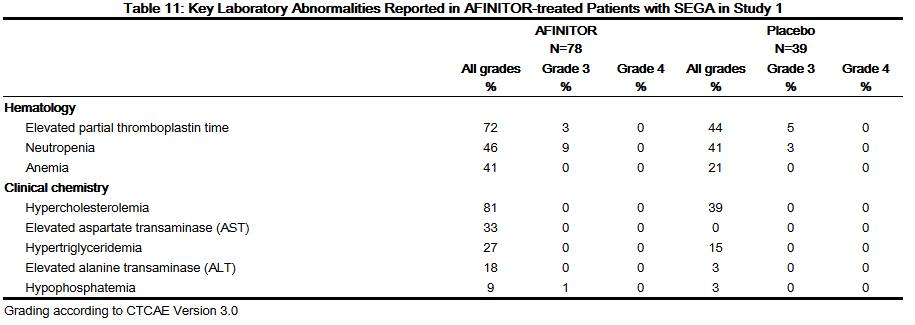
- The most common adverse reactions reported for AFINITOR (incidence ≥ 30%) were stomatitis and respiratory tract infection. The most common Grade 3-4 adverse reactions (incidence ≥ 2%) were stomatitis, pyrexia, pneumonia, gastroenteritis, aggression, agitation, and amenorrhea. The most common key laboratory abnormalities (incidence ≥ 50%) were hypercholesterolemia and elevated partial thromboplastin time. The most common Grade 3-4 laboratory abnormality (incidence ≥ 3%) was neutropenia.
- There were no adverse reactions resulting in permanent discontinuation. Dose adjustments (interruptions or reductions) due to adverse reactions occurred in 55% of AFINITOR-treated patients. The most common adverse reaction leading to AFINITOR dose adjustment was stomatitis.
- Table 10 compares the incidence of adverse reactions reported with an incidence of ≥ 10% for patients receiving AFINITOR and occurring more frequently with AFINITOR than with placebo. Laboratory abnormalities are described separately in Table 11.
- Amenorrhea occurred in 17% of AFINITOR-treated females aged 10 to 55 years (3 of 18) and none of the females in the placebo group. For this same group of AFINITOR-treated females, the following menstrual abnormalities were reported: dysmenorrhea (6%), menorrhagia (6%), metrorrhagia (6%), and unspecified menstrual irregularity (6%).
- The following additional adverse reactions occurred in less than 10% of AFINITOR-treated patients: nausea (8%), pain in extremity (8%), insomnia (6%), pneumonia (6%), epistaxis (5%), hypersensitivity (3%), increased blood luteinizing hormone (LH) levels (1%) and pneumonitis (1%).
- Longer-term follow-up of 34.2 months (range 4.7 to 47.1 months) from a non-randomized, open-label, 28-patient trial resulted in the following additional notable adverse reactions and key laboratory abnormalities: cellulitis (29%), hyperglycemia (25%), and elevated creatinine (14%).
Postmarketing Experience
- The following adverse reactions have been identified during post approval use of AFINITOR. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate frequency or establish a causal relationship to drug exposure: acute pancreatitis, cholecystitis, cholelithiasis, arterial thrombotic events and reflex sympathetic dystrophy.
Drug Interactions
- Agents That May Increase Everolimus Blood Concentrations
- CYP3A4 Inhibitors and PgP Inhibitors
- In healthy subjects, compared to AFINITOR treatment alone there were significant increases in everolimus exposure when AFINITOR was coadministered with:
- ketoconazole (a strong CYP3A4 inhibitor and a PgP inhibitor) - Cmax and AUC increased by 3.9- and 15.0-fold, respectively.
- erythromycin (a moderate CYP3A4 inhibitor and a PgP inhibitor) - Cmax and AUC increased by 2.0- and 4.4-fold, respectively.
- verapamil (a moderate CYP3A4 inhibitor and a PgP inhibitor) - Cmax and AUC increased by 2.3- and 3.5-fold, respectively.
- In healthy subjects, compared to AFINITOR treatment alone there were significant increases in everolimus exposure when AFINITOR was coadministered with:
- CYP3A4 Inhibitors and PgP Inhibitors
- Concomitant strong inhibitors of CYP3A4/PgP should not be used.
- Use caution when AFINITOR is used in combination with moderate CYP3A4/PgP inhibitors. If alternative treatment cannot be administered reduce the AFINITOR dose.
- Agents That May Decrease Everolimus Blood Concentrations
- CYP3A4/PgP Inducers
- In healthy subjects, co-administration of AFINITOR with rifampin, a strong inducer of CYP3A4 and an inducer of PgP, decreased everolimus AUC and Cmax by 63% and 58% respectively, compared to everolimus treatment alone. Consider a dose increase of AFINITOR when co-administered with strong CYP3A4/PgP inducers if alternative treatment cannot be administered. St. John’s Wort may decrease everolimus exposure unpredictably and should be avoided.
- CYP3A4/PgP Inducers
- Drugs That May Have Their Plasma Concentrations Altered by Everolimus
- Studies in healthy subjects indicate that there are no clinically significant pharmacokinetic interactions between AFINITOR and the HMG-CoA reductase inhibitors atorvastatin (a CYP3A4 substrate) and pravastatin (a non-CYP3A4 substrate) and population pharmacokinetic analyses also detected no influence of simvastatin (a CYP3A4 substrate) on the clearance of AFINITOR.
- A study in healthy subjects demonstrated that co-administration of an oral dose of midazolam (sensitive CYP3A4 substrate) with everolimus resulted in a 25% increase in midazolam Cmax and a 30% increase in midazolam AUC(0-inf).
- Coadministration of everolimus and exemestane increased exemestane Cmin by 45% and C2h by 64%. However, the corresponding estradiol levels at steady state (4 weeks) were not different between the 2 treatment arms. No increase in adverse events related to exemestane was observed in patients with hormone receptor-positive, HER2-negative advanced breast cancer receiving the combination.
- Coadministration of everolimus and depot octreotide increased octreotide Cmin by approximately 50%.
Use in Specific Populations
Pregnancy
- Pregnancy Category D
- Risk Summary
- Based on the mechanism of action, AFINITOR can cause fetal harm when administered to a pregnant woman. Everolimus caused embryo-fetal toxicities in animals at maternal exposures that were lower than human exposures. If this drug is used during pregnancy or if the patient becomes pregnant while taking the drug, apprise the patient of the potential hazard to the fetus.
- Animal Data
- In animal reproductive studies, oral administration of everolimus to female rats before mating and through organogenesis induced embryo-fetal toxicities, including increased resorption, pre-implantation and post-implantation loss, decreased numbers of live fetuses, malformation (e.g., sternal cleft), and retarded skeletal development. These effects occurred in the absence of maternal toxicities. Embryo-fetal toxicities in rats occurred at doses ≥ 0.1 mg/kg (0.6 mg/m2) with resulting exposures of approximately 4% of the exposure (AUC0-24h) achieved in patients receiving the 10 mg daily dose of everolimus. In rabbits, embryotoxicity evident as an increase in resorptions occurred at an oral dose of 0.8 mg/kg (9.6 mg/m2), approximately 1.6 times either the 10 mg daily dose or the median dose administered to SEGA patients on a body surface area basis. The effect in rabbits occurred in the presence of maternal toxicities.
- In a pre- and post-natal development study in rats, animals were dosed from implantation through lactation. At the dose of 0.1 mg/kg (0.6 mg/m2), there were no adverse effects on delivery and lactation or signs of maternal toxicity; however, there were reductions in body weight (up to 9% reduction from the control) and in survival of offspring (~5% died or missing). There were no drug-related effects on the developmental parameters (morphological development, motor activity, learning, or fertility assessment) in the offspring.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Everolimus in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Everolimus during labor and delivery.
Nursing Mothers
- It is not known whether everolimus is excreted in human milk. Everolimus and/or its metabolites passed into the milk of lactating rats at a concentration 3.5 times higher than in maternal serum. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from everolimus, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- Pediatric use of AFINITOR Tablets and AFINITOR DISPERZ is recommended for patients 1 year of age and older with TSC for the treatment of SEGA that requires therapeutic intervention but cannot be curatively resected. The safety and effectiveness of AFINITOR Tablets and AFINITOR DISPERZ have not been established in pediatric patients with renal angiomyolipoma with TSC in the absence of SEGA.
- The effectiveness of AFINITOR in pediatric patients with SEGA was demonstrated in two clinical trials based on demonstration of durable objective response, as evidenced by reduction in SEGA tumor volume. Improvement in disease-related symptoms and overall survival in pediatric patients with SEGA has not been demonstrated. The long term effects of AFINITOR on growth and pubertal development are unknown.
- Study 1 was a randomized, double-blind, multicenter trial comparing AFINITOR (n=78) to placebo (n=39) in pediatric and adult patients. The median age was 9.5 years (range 0.8 to 26 years). At the time of randomization, a total of 20 patients were < 3 years of age, 54 patients were 3 to < 12 years of age, 27 patients were 12 to < 18 years of age, and 16 patients were ≥ 18 years of age. The overall nature, type, and frequency of adverse reactions across the age groups evaluated were similar, with the exception of a higher per patient incidence of infectious serious adverse events in patients < 3 years of age. A total of 6 of 13 patients (46%) < 3 years of age had at least 1 serious adverse event due to infection, compared to 2 of 7 patients (29%) treated with placebo. No patient in any age group discontinued AFINITOR due to infection. Subgroup analyses showed reduction in SEGA volume with AFINITOR treatment in all pediatric age subgroups.
- Study 2 was an open-label, single-arm, single-center trial of AFINITOR (N=28) in patients aged ≥ 3 years; median age was 11 years (range 3 to 34 years). A total of 16 patients were 3 to < 12 years, 6 patients were 12 to < 18 years, and 6 patients were ≥ 18 years. The frequency of adverse reactions across the age groups was generally similar. Subgroup analyses showed reductions in SEGA volume with AFINITOR treatment in all pediatric age subgroups.
- Everolimus clearance normalized to body surface area was higher in pediatric patients than in adults with SEGA. The recommended starting dose and subsequent requirement for therapeutic drug monitoring to achieve and maintain trough concentrations of 5 to 15 ng/mL are the same for adult and pediatric patients with SEGA.
Geriatic Use
- In the randomized advanced hormone receptor positive, HER2-negative breast cancer study, 40% of AFINITOR-treated patients were ≥ 65 years of age, while 15% were 75 years and over. No overall differences in effectiveness were observed between elderly and younger patients. The incidence of deaths due to any cause within 28 days of the last AFINITOR dose was 6% in patients ≥ 65 years of age compared to 2% in patients < 65 years of age. Adverse reactions leading to permanent treatment discontinuation occurred in 33% of patients ≥ 65 years of age compared to 17% in patients < 65 years of age.
- In two other randomized trials (advanced renal cell carcinoma and advanced neuroendocrine tumors of pancreatic origin), no overall differences in safety or effectiveness were observed between elderly and younger patients. In the randomized advanced RCC study, 41% of AFINITOR treated patients were ≥ 65 years of age, while 7% were 75 years and over. In the randomized advanced PNET study, 30% of AFINITOR-treated patients were ≥ 65 years of age, while 7% were 75 years and over.
- Other reported clinical experience has not identified differences in response between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
- No dosage adjustment in initial dosing is required in elderly patients, but close monitoring and appropriate dose adjustments for adverse reactions is recommended.
Gender
There is no FDA guidance on the use of Everolimus with respect to specific gender populations.
Race
There is no FDA guidance on the use of Everolimus with respect to specific racial populations.
Renal Impairment
- No clinical studies were conducted with AFINITOR in patients with decreased renal function. Renal impairment is not expected to influence drug exposure and no dosage adjustment of everolimus is recommended in patients with renal impairment.
Hepatic Impairment
- The safety, tolerability and pharmacokinetics of AFINITOR were evaluated in a 34 subject single oral dose study of everolimus in subjects with impaired hepatic function relative to subjects with normal hepatic function. Exposure was increased in patients with mild (Child-Pugh class A), moderate (Child-Pugh class B), and severe (Child-Pugh class C) hepatic impairment.
- For advanced HR+ BC, advanced PNET, advanced RCC, and renal angiomyolipoma with TSC patients with severe hepatic impairment, AFINITOR may be used at a reduced dose if the desired benefit outweighs the risk. For patients with mild (Child-Pugh class A) or moderate (Child-Pugh class B) hepatic impairment, a dose reduction is recommended.
- For patients with SEGA who have severe hepatic impairment (Child-Pugh class C), reduce the starting dose of AFINITOR Tablets or AFINITOR DISPERZ by approximately 50%. For patients with SEGA who have mild (Child-Pugh class A) or moderate (Child-Pugh class B) hepatic impairment, adjustment to the starting dose may not be needed. Subsequent dosing should be based on therapeutic drug monitoring.
Females of Reproductive Potential and Males
- Contraception
- Females
- AFINITOR can cause fetal harm when administered to a pregnant woman. Advise female patients of reproductive potential to use highly effective contraception while receiving AFINITOR and for up to 8 weeks after ending treatment.
- Females
- Infertility
- Females
- Menstrual irregularities, secondary amenorrhea, and increases in luteinizing hormone (LH) and follicle stimulating hormone (FSH) occurred in female patients taking AFINITOR. Based on these clinical findings and findings in animals, female fertility may be compromised by treatment with AFINITOR.
- Females
- Males
- AFINITOR treatment may impair fertility in male patients based on animal findings.
- Males
Immunocompromised Patients
There is no FDA guidance one the use of Everolimus in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
- Renal function of patients should be monitored particularly where patients have additional risk factors that may further impair renal function.
- Monitoring of fasting serum glucose and lipid profile is recommended prior to the start of AFINITOR therapy and periodically thereafter as well as management with appropriate medical therapy.
- Monitoring of complete blood count is recommended prior to the start of AFINITOR therapy and periodically thereafter.
IV Compatibility
There is limited information regarding IV Compatibility of Everolimus in the drug label.
Overdosage
Acute Overdose
- In animal studies, everolimus showed a low acute toxic potential. No lethality or severe toxicity was observed in either mice or rats given single oral doses of 2000 mg/kg (limit test).
- Reported experience with overdose in humans is very limited. Single doses of up to 70 mg have been administered. The acute toxicity profile observed with the 70 mg dose was consistent with that for the 10 mg dose.
Chronic Overdose
There is limited information regarding Chronic Overdose of Everolimus in the drug label.
Pharmacology
There is limited information regarding Everolimus Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Everolimus Mechanism of Action in the drug label.
Structure
There is limited information regarding Everolimus Structure in the drug label.
Pharmacodynamics
There is limited information regarding Everolimus Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Everolimus Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Everolimus Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Everolimus Clinical Studies in the drug label.
How Supplied
There is limited information regarding Everolimus How Supplied in the drug label.
Storage
There is limited information regarding Everolimus Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Everolimus |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Everolimus |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Everolimus Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Everolimus interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Everolimus Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Everolimus Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.