Etodolac
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Deepika Beereddy, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Cardiovascular Risk & Gastrointestinal Risk
See full prescribing information for complete Boxed Warning.
Cardiovascular Risk:
|
Overview
Etodolac is an analgesic, anti-inflammatory agent that is FDA approved for the treatment of juvenile arthritis, rheumatoid arthritis, osteoarthritis. There is a Black Box Warning for this drug as shown here. Common adverse reactions include edema, abdominal pain, dairrhea, flatulance, indigestion, nausea, increased liver function test, dizziness, headache, malaise.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Osteoarthritis
- Dosing Information
- For the relief of the signs and symptoms of osteoarthritis, the recommended starting dose of etodolac extended-release tablets is 400 to 1000 mg given orally once per day.
- As with other NSAIDs, the lowest effective dose should be sought for each patient. In chronic conditions, a therapeutic response to therapy with etodolac extended-release tablets is sometimes seen within one week of therapy, but most often is observed by two weeks.
Pain
- Dosing Information
- Immediate release, 200 to 400 mg ORALLY every 6 to 8 h as needed; max 1200 mg/day
Rheumatoid arthritis
- Dosing Information
- For the relief of the signs and symptoms of rheumatoid arthritis, the recommended starting dose of etodolac extended-release tablets is 400 to 1000 mg given orally once per day.
- As with other NSAIDs, the lowest effective dose should be sought for each patient. In chronic conditions, a therapeutic response to therapy with etodolac extended-release tablets is sometimes seen within one week of therapy, but most often is observed by two weeks.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- Gout
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Juvenile rheumatoid arthritis
- Dosing Information
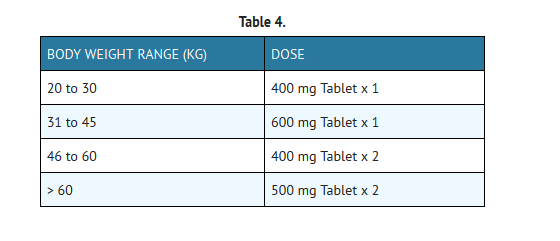
- For the relief of the signs and symptoms of juvenile rheumatoid arthritis in patients 6 to 16 years of age, the recommended dose given orally once per day should be based on body weight, according to the following table:
Off-Label Use and Dosage (Pediatric)
Non–Guideline-Supported Use
- Rheumatoid arthritis
Contraindications
- Condition1
Warnings
|
Cardiovascular Risk & Gastrointestinal Risk
See full prescribing information for complete Boxed Warning.
Cardiovascular Risk:
|
- Description
Precautions
- Description
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Etodolac in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Etodolac in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Etodolac in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Etodolac during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Etodolac with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Etodolac with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Etodolac with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Etodolac with respect to specific gender populations.
Race
There is no FDA guidance on the use of Etodolac with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Etodolac in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Etodolac in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Etodolac in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Etodolac in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Etodolac in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Etodolac in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Etodolac in the drug label.
Pharmacology
There is limited information regarding Etodolac Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Etodolac Mechanism of Action in the drug label.
Structure
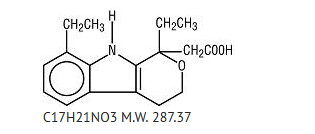
- Etodolac extended-release tablets contain etodolac, which is a member of the pyranocarboxylic acid group of non-steroidal anti-inflammatory drugs (NSAIDs). Each tablet contains etodolac for oral administration. Etodolac is a racemic mixture of [+]S and [-]R-enantiomers. It is a white crystalline compound, insoluble in water but soluble in alcohols, chloroform, dimethyl sulfoxide, and aqueous polyethylene glycol.
- The chemical name is (±) 1,8-diethyl-1,3,4,9-tetrahydropyrano-[3,4-b]indole-1-acetic acid. It has the following structural formula:
- The inactive ingredients in etodolac extended-release tablets are calcium phosphate dibasic anhydrous, carbomer 934P, colloidal silicon dioxide, hydroxypropyl cellulose, hypromellose, lactose monohydrate, magnesium stearate, polyethylene glycol, sodium lauryl sulfate, and titanium dioxide. In addition, the following colorants are used: 400 mg tablets - D&C Yellow #10 Lake, FD&C Red #40, and FD&C Yellow #6; 500 mg tablets - D&C Yellow #10 Lake, FD&C Blue No. 2 Indigo Carmine Aluminum Lake, and iron oxide black; 600 mg tablets - FD&C Blue No. 2 Indigo Carmine Lake, iron oxide black, and iron oxide yellow.
Pharmacodynamics
- Etodolac extended-release tablets are a non-steroidal anti-inflammatory drug (NSAID) that exhibits anti-inflammatory, analgesic, and antipyretic activities in animal models. The mechanism of action of etodolac extended-release tablets, like that of other NSAIDs, is not completely understood, but may be related to prostaglandin synthetase inhibition.
Pharmacokinetics
Absorption
- Etodolac extended-release tablets and etodolac tablets both contain etodolac, but differ in their release characteristics. The systemic availability of etodolac from etodolac extended-release tablets is generally greater than 80%. Etodolac does not undergo significant first-pass metabolism following oral administration. After oral administration of etodolac extended-release tablets in doses up to 800 mg once daily, peak concentrations occur approximately 6 hours after dosing and are dose proportional for both total and free etodolac.
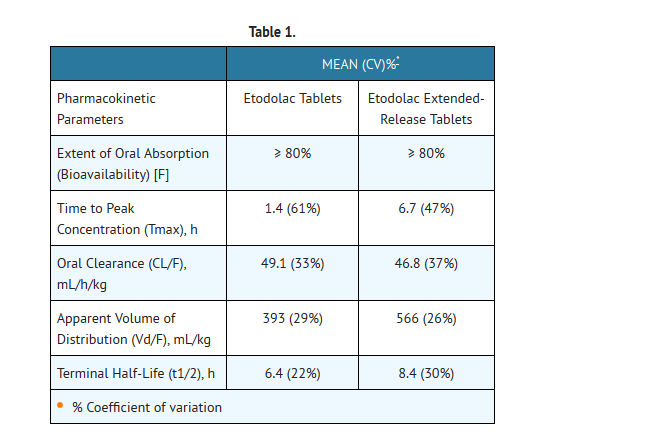
- Table 1 shows the comparison of etodolac pharmacokinetic parameters after the administration of etodolac tablets and etodolac extended-release tablets.
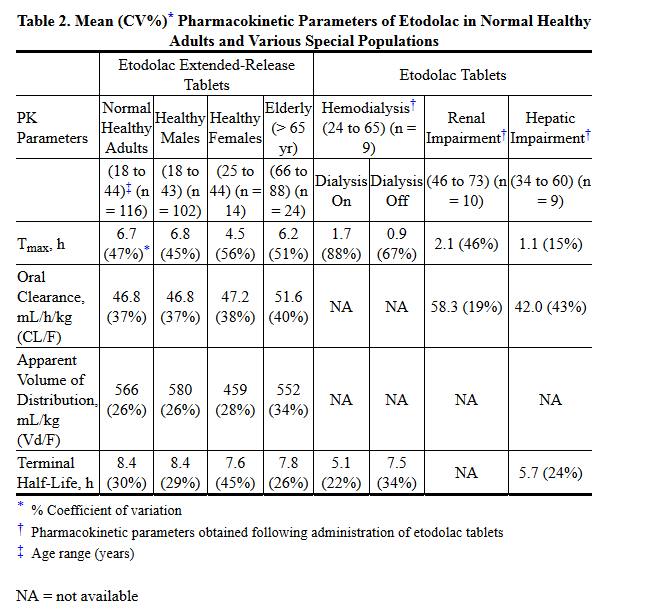
- Table 2 shows the etodolac pharmacokinetic parameters in various populations. The data from patients with renal and hepatic impairment were obtained following administration of (immediate-release) etodolac tablets.
Food/Antacid Effects
- Food has no significant effect on the extent of etodolac extended-release tablets absorption, however, food significantly increased Cmax (54%) following a 600 mg dose.
- The extent of absorption of etodolac is not affected when etodolac is administered with antacid. Coadministration, with an antacid, decreases the peak concentration reached by about 15 to 20% with no measurable effect on time-to-peak.
Distribution
- The mean apparent volume of distribution (Vd/F) of etodolac following administration of etodolac extended-release tablets is 566 mL/kg. Etodolac is more than 99% bound to plasma proteins, primarily to albumin, and is independent of etodolac concentration over the dose range studied. It is not known whether etodolac is excreted in human milk. However, based on its physical-chemical properties, excretion into breast milk is expected.
Metabolism
- Etodolac metabolites do not contribute significantly to the pharmacological activity of etodolac extended-release tablets.
- Following administration of immediate-release etodolac, several metabolites have been identified in human plasma and urine. Other metabolites remain to be identified. The metabolites include 6-, 7-, and 8-hydroxylated etodolac and etodolac glucuronide. After a single dose of 14C-etodolac, hydroxylated metabolites accounted for less than 10% of total drug in serum. On chronic dosing, hydroxylated-etodolac metabolites do not accumulate in the plasma of patients with normal renal function. The extent of accumulation of hydroxylated-etodolac metabolites in patients with renal dysfunction has not been studied. The role, if any, of a specific cytochrome P450 system in the metabolism of etodolac is unknown. The hydroxylated-etodolac metabolites undergo further glucuronidation followed by renal excretion and partial elimination in the feces.
Excretion
- The mean oral clearance of etodolac following oral etodolac extended-release tablets dosing is 47 (±17) mL/h/kg. The terminal half-life (t1/2) of etodolac after etodolac extended-release tablets administration is 8.4 hours compared to 6.4 hours for etodolac tablets. Approximately 1% of an etodolac tablet dose is excreted unchanged in the urine, with 72% of the dose excreted into the urine as parent drug plus metabolites:
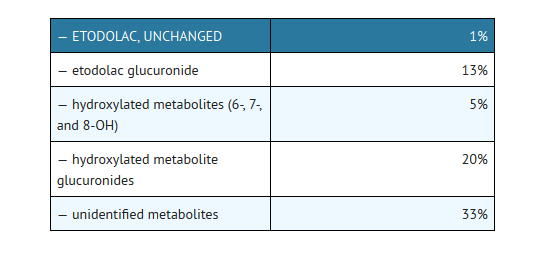
- Fecal excretion accounted for 16% of the dose.
Special Populations
Geriatric
- In clinical studies, age was not shown to have any effect on half-life or protein binding, and demonstrated no change in expected drug accumulation. No dosage adjustment is generally necessary in the elderly on the basis of pharmacokinetics. The elderly may need dosage adjustment, however, as they may be more sensitive to antiprostaglandin effects than younger patients.
Pediatric
- The pharmacokinetics of etodolac extended-release tablets were assessed in an open-label, 12 week clinical trial which included plasma sampling for population pharmacokinetics. Seventy-two (72) patients, 6 to 16 years of age, with juvenile rheumatoid arthritis, received etodolac extended-release tablets in doses of 13.3 to 21.3 mg/kg given as 400 to 1000 mg once daily. The results from a population pharmacokinetic analysis based on the 59 subjects who completed the trial are as follows:
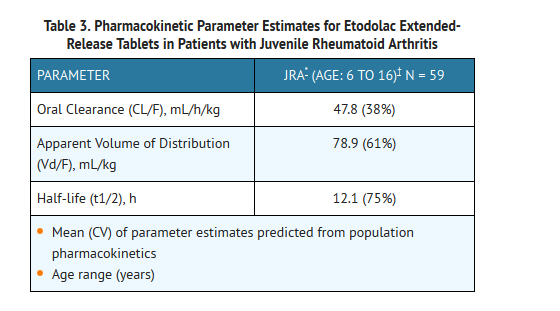
- While similar, the pharmacokinetic parameters for children with juvenile rheumatoid arthritis did not directly correlate with adult pharmacokinetic data in rheumatoid arthritis. In the population pharmacokinetic analysis, body weights below 50 kg were found to correlate with CL/F.
Race
- Pharmacokinetic differences due to race have not been identified. Clinical studies included patients of many races, all of whom responded in a similar fashion.
Hepatic Insufficiency
- The pharmacokinetics of etodolac following administration of etodolac extended-release tablets have not been investigated in subjects with hepatic insufficiency. Following administration of etodolac tablets, the plasma protein binding and disposition of total and free etodolac were unchanged in the presence of compensated hepatic cirrhosis. Although no dosage adjustment is generally required in patients with chronic hepatic diseases, etodolac clearance is dependent on liver function and could be reduced in patients with severe hepatic failure.
Renal Insufficiency
- The pharmacokinetics of etodolac following administration of etodolac extended-release tablets have not been investigated in subjects with renal insufficiency. Etodolac renal clearance following administration of etodolac tablets was unchanged in the presence of mild-to-moderate renal failure (creatinine clearance, 37 to 88 mL/min). Although renal elimination is a significant pathway of excretion for etodolac metabolites, no dosing adjustment in patients with mild to moderate renal dysfunction is generally necessary. Etodolac plasma protein binding decreases in patients with severe renal deficiency. Etodolac should be used with caution in such patients because, as with other NSAIDs, it may further decrease renal function in some patients. Etodolac is not significantly removed from the blood in patients undergoing hemodialysis.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Etodolac in the drug label.
Clinical Studies
Arthritis
- The use of etodolac extended-release tablets in managing the signs and symptoms of osteoarthritis of the knee and rheumatoid arthritis was assessed in double-blind, randomized, parallel, controlled clinical trials in 1552 patients. In these trials, etodolac extended-release tablets, given once daily, provided efficacy comparable to immediate-release etodolac.
- The safety, efficacy, and pharmacokinetics of etodolac extended-release tablets were assessed in an open-label, 12 week clinical trial. Seventy-two (72) patients, 6 to 16 years of age, with juvenile rheumatoid arthritis, received etodolac extended-release tablets in doses of 400 to 1000 mg (13.3 to 21.3 mg/kg body weight) once daily. At these doses, etodolac extended-release tablets controlled the signs and symptoms of juvenile rheumatoid arthritis. Based on the results of this study, the safety profile of etodolac extended-release tablets (at doses not exceeding 20 mg/kg) appeared to be similar to that observed in the adult arthritic patients in clinical trials.
How Supplied
Storage
There is limited information regarding Etodolac Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Etodolac |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Etodolac |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Etodolac in the drug label.
Precautions with Alcohol
- Alcohol-Etodolac interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Lodine, Lodine XL
Look-Alike Drug Names
- A® — B®[1]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Etodolac
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Etodolac |Label Name=Etodolac11.png
}}
{{#subobject:
|Label Page=Etodolac |Label Name=Etodolac11.png
}}