Ertugliflozin: Difference between revisions
(Created page with "{{DrugProjectFormSinglePage |authorTag= {{Sonya}} |genericName=generic name |aOrAn=a |drugClass=Acetylcholine release inhibitor, Adrenergic receptor agonist |indicationType=(t...") |
No edit summary |
||
| Line 313: | Line 313: | ||
(Description) | (Description) | ||
|howSupplied= | |howSupplied= | ||
|storage=( | *Ertugliflozin tablets are available in the strengths listed below: | ||
*5 mg tablets, are pink, triangular-shaped, biconvex, with "701" debossed on one side and plain on the other side. They are supplied as follows: | |||
:*NDC 0006-5363-03 unit-of-use bottles of 30 | |||
:*NDC 0006-5363-06 unit-of-use bottles of 90 | |||
:*NDC 0006-5363-07 bulk bottles of 500 | |||
:*15 mg tablets, are red, triangular-shaped, biconvex, with "702" debossed on one side and plain on the other side. They are supplied as follows: | |||
:*NDC 0006-5364-03 unit-of-use bottles of 30 | |||
:*NDC 0006-5364-06 unit-of-use bottles of 90 | |||
:*NDC 0006-5364-07 bulk bottles of 500 | |||
|storage= | |||
*Store at 20°C -25°C (68°F -77°F), excursions permitted between 15°C -30°C (between 59°F -86°F). Protect from moisture. Store in a dry place. | |||
|packLabel= | |packLabel= | ||
|fdaPatientInfo=( | [[image:steglatrolabel1.jpeg|none|thumb|400px|This image is provided by the National Library of Medicine.]] | ||
[[image:steglatrolabel2.jpeg|none|thumb|400px|This image is provided by the National Library of Medicine.]] | |||
|fdaPatientInfo= | |||
* ( | *Advise the patient to read the FDA-approved patient labeling (Medication Guide). | ||
* ( | =====Instructions===== | ||
*Instruct patients to read the Medication Guide before starting ertugliflozin and to reread it each time the prescription is renewed. | |||
*Inform patients of the potential risks and benefits of ertugliflozin and of alternative modes of therapy. Also inform patients about the importance of adherence to dietary instructions, regular physical activity, periodic blood glucose monitoring and HbA1c testing, recognition and management of hypoglycemia and hyperglycemia, and assessment for diabetes complications. Advise patients to seek medical advice promptly during periods of stress such as fever, trauma, infection, or surgery, as medication requirements may change. | |||
*Instruct patients to take ertugliflozin only as prescribed. If a dose is missed, advise patients to take it as soon as it is remembered unless it is almost time for the next dose, in which case patients should skip the missed dose and take the medicine at the next regularly scheduled time. Advise patients not to take two doses of ertugliflozin at the same time. | |||
=====Hypoglycemia with Concomitant Use of Insulin and/or Insulin Secretagogue===== | |||
*Inform patients that the incidence of hypoglycemia may increase when ertugliflozin is added to insulin and/or an insulin secretagogue and that a lower dose of insulin or insulin secretagogue may be required to reduce the risk of hypoglycemia | |||
=====Hypotension===== | |||
*Inform patients that symptomatic hypotension may occur with ertugliflozin and advise them to contact their doctor if they experience such symptoms. Inform patients that dehydration may increase the risk for hypotension, and to have adequate fluid intake. | |||
=====Ketoacidosis===== | |||
*Inform patients that ketoacidosis is a serious life-threatening condition. Cases of ketoacidosis have been reported during use of ertugliflozin. Instruct patients to check ketones (when possible) if symptoms consistent with ketoacidosis occur even if blood glucose is not elevated. If symptoms of ketoacidosis (including nausea, vomiting, abdominal pain, tiredness, and labored breathing) occur, instruct patients to discontinue ertugliflozin and seek medical advice immediately. | |||
=====Acute Kidney Injury===== | |||
*Inform patients that acute kidney injury has been reported during use of ertugliflozin. Advise patients to seek medical advice immediately if they have reduced oral intake (due to acute illness or fasting) or increased fluid losses (due to vomiting, diarrhea, or excessive heat exposure), as it may be appropriate to temporarily discontinue ertugliflozin use in those settings. | |||
=====Monitoring of Renal Function===== | |||
*Inform patients about the importance of regular testing of renal function when receiving treatment with ertugliflozin. | |||
=====Serious Urinary Tract Infections===== | |||
*Inform patients of the potential for urinary tract infections, which may be serious. Provide them with information on the symptoms of urinary tract infections. Advise them to seek medical advice if such symptoms occur. | |||
=====Amputation===== | |||
*Inform patients of the potential for an increased risk of amputations. Counsel patients about the importance of routine preventative foot care. Instruct patients to monitor for new pain or tenderness, sores or ulcers, or infections involving the leg or foot and to seek medical advice immediately if such signs or symptoms develop. | |||
=====Genital Mycotic Infections in Females (e.g., Vulvovaginitis)===== | |||
*Inform female patients that vaginal yeast infections may occur and provide them with information on the signs and symptoms of vaginal yeast infection. Advise them of treatment options and when to seek medical advice. | |||
=====Genital Mycotic Infections in Males (e.g., Balanitis or Balanoposthitis)===== | |||
*Inform male patients that yeast infections of the penis (e.g., balanitis or balanoposthitis) may occur, especially in uncircumcised males. Provide them with information on the signs and symptoms of balanitis and balanoposthitis (rash or redness of the glans or foreskin of the penis). Advise them of treatment options and when to seek medical advice. | |||
=====Fetal Toxicity===== | |||
*Advise pregnant patients of the potential risk to a fetus with treatment with ertugliflozin. Instruct patients to immediately inform their healthcare provider if pregnant or planning to become pregnant. | |||
=====Lactation===== | |||
*Advise patients that use of ertugliflozin is not recommended while breastfeeding. | |||
=====Laboratory Tests===== | |||
*Due to its mechanism of action, inform patients that their urine will test positive for glucose while taking ertugliflozin. | |||
[[image:ertugliflozinmedguide.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | |||
|nlmPatientInfo= | |||
|lookAlike= | |||
|brandNames= | |brandNames= | ||
*Steglatro | |||
|drugShortage=Drug Shortage | |drugShortage=Drug Shortage | ||
}} | }} | ||
Revision as of 18:20, 16 July 2018
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sonya Gelfand
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Warning Title
See full prescribing information for complete Boxed Warning.
Condition Name: (Content)
|
Overview
Ertugliflozin is a Acetylcholine release inhibitor, Adrenergic receptor agonist that is FDA approved for the (type of indication of drug) of a list of indications, separated by commas.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include a list of adverse reactions, separated by commas..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
CONTRAINDICATIONS
Warnings
|
Warning Title
See full prescribing information for complete Boxed Warning.
Condition Name: (Content)
|
Conidition 1
(Description)
Conidition 2
(Description)
Conidition 3
(Description)
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Condition 2
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Postmarketing Experience
(Description)
Drug Interactions
- Drug 1
- Drug 2
- Drug 3
- Drug 4
- Drug 5
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description)
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
(Description)
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ertugliflozin in women who are pregnant.
Labor and Delivery
(Description)
Nursing Mothers
(Description)g
Pediatric Use
(Description)
Geriatic Use
(Description)
Gender
(Description)
Race
(Description)
Renal Impairment
(Description)
Hepatic Impairment
(Description)
Females of Reproductive Potential and Males
(Description)
Immunocompromised Patients
(Description)
Others
(Description)
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
There is limited information regarding the compatibility of Ertugliflozin and IV administrations.
Overdosage
Acute Overdose
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description)
Pharmacology
Ertugliflozin
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
(Description)
Structure
(Description with picture)
Pharmacodynamics
(Description)
Pharmacokinetics
(Description)
Nonclinical Toxicology
(Description)
Clinical Studies
Condition 1
(Description)
Condition 2
(Description)
Condition 3
(Description)
How Supplied
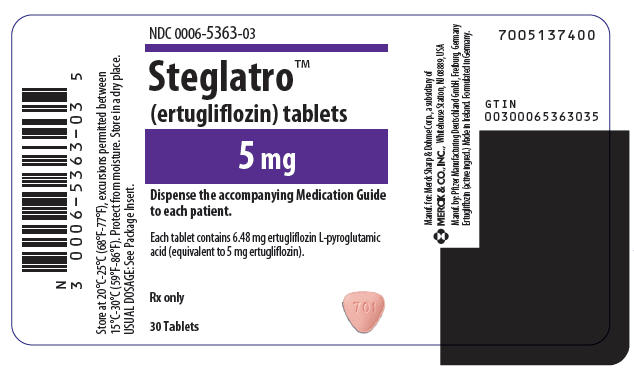
- Ertugliflozin tablets are available in the strengths listed below:
- 5 mg tablets, are pink, triangular-shaped, biconvex, with "701" debossed on one side and plain on the other side. They are supplied as follows:
- NDC 0006-5363-03 unit-of-use bottles of 30
- NDC 0006-5363-06 unit-of-use bottles of 90
- NDC 0006-5363-07 bulk bottles of 500
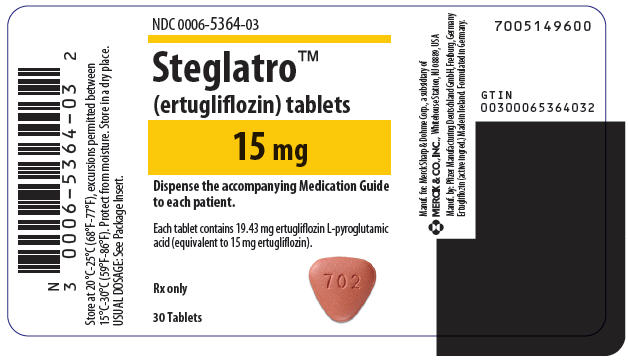
- 15 mg tablets, are red, triangular-shaped, biconvex, with "702" debossed on one side and plain on the other side. They are supplied as follows:
- NDC 0006-5364-03 unit-of-use bottles of 30
- NDC 0006-5364-06 unit-of-use bottles of 90
- NDC 0006-5364-07 bulk bottles of 500
Storage
- Store at 20°C -25°C (68°F -77°F), excursions permitted between 15°C -30°C (between 59°F -86°F). Protect from moisture. Store in a dry place.
Images
Drug Images
{{#ask: Page Name::Ertugliflozin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Ertugliflozin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Instructions
- Instruct patients to read the Medication Guide before starting ertugliflozin and to reread it each time the prescription is renewed.
- Inform patients of the potential risks and benefits of ertugliflozin and of alternative modes of therapy. Also inform patients about the importance of adherence to dietary instructions, regular physical activity, periodic blood glucose monitoring and HbA1c testing, recognition and management of hypoglycemia and hyperglycemia, and assessment for diabetes complications. Advise patients to seek medical advice promptly during periods of stress such as fever, trauma, infection, or surgery, as medication requirements may change.
- Instruct patients to take ertugliflozin only as prescribed. If a dose is missed, advise patients to take it as soon as it is remembered unless it is almost time for the next dose, in which case patients should skip the missed dose and take the medicine at the next regularly scheduled time. Advise patients not to take two doses of ertugliflozin at the same time.
Hypoglycemia with Concomitant Use of Insulin and/or Insulin Secretagogue
- Inform patients that the incidence of hypoglycemia may increase when ertugliflozin is added to insulin and/or an insulin secretagogue and that a lower dose of insulin or insulin secretagogue may be required to reduce the risk of hypoglycemia
Hypotension
- Inform patients that symptomatic hypotension may occur with ertugliflozin and advise them to contact their doctor if they experience such symptoms. Inform patients that dehydration may increase the risk for hypotension, and to have adequate fluid intake.
Ketoacidosis
- Inform patients that ketoacidosis is a serious life-threatening condition. Cases of ketoacidosis have been reported during use of ertugliflozin. Instruct patients to check ketones (when possible) if symptoms consistent with ketoacidosis occur even if blood glucose is not elevated. If symptoms of ketoacidosis (including nausea, vomiting, abdominal pain, tiredness, and labored breathing) occur, instruct patients to discontinue ertugliflozin and seek medical advice immediately.
Acute Kidney Injury
- Inform patients that acute kidney injury has been reported during use of ertugliflozin. Advise patients to seek medical advice immediately if they have reduced oral intake (due to acute illness or fasting) or increased fluid losses (due to vomiting, diarrhea, or excessive heat exposure), as it may be appropriate to temporarily discontinue ertugliflozin use in those settings.
Monitoring of Renal Function
- Inform patients about the importance of regular testing of renal function when receiving treatment with ertugliflozin.
Serious Urinary Tract Infections
- Inform patients of the potential for urinary tract infections, which may be serious. Provide them with information on the symptoms of urinary tract infections. Advise them to seek medical advice if such symptoms occur.
Amputation
- Inform patients of the potential for an increased risk of amputations. Counsel patients about the importance of routine preventative foot care. Instruct patients to monitor for new pain or tenderness, sores or ulcers, or infections involving the leg or foot and to seek medical advice immediately if such signs or symptoms develop.
Genital Mycotic Infections in Females (e.g., Vulvovaginitis)
- Inform female patients that vaginal yeast infections may occur and provide them with information on the signs and symptoms of vaginal yeast infection. Advise them of treatment options and when to seek medical advice.
Genital Mycotic Infections in Males (e.g., Balanitis or Balanoposthitis)
- Inform male patients that yeast infections of the penis (e.g., balanitis or balanoposthitis) may occur, especially in uncircumcised males. Provide them with information on the signs and symptoms of balanitis and balanoposthitis (rash or redness of the glans or foreskin of the penis). Advise them of treatment options and when to seek medical advice.
Fetal Toxicity
- Advise pregnant patients of the potential risk to a fetus with treatment with ertugliflozin. Instruct patients to immediately inform their healthcare provider if pregnant or planning to become pregnant.
Lactation
- Advise patients that use of ertugliflozin is not recommended while breastfeeding.
Laboratory Tests
- Due to its mechanism of action, inform patients that their urine will test positive for glucose while taking ertugliflozin.
Precautions with Alcohol
Alcohol-Ertugliflozin interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Steglatro
Look-Alike Drug Names
There is limited information regarding Ertugliflozin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.