Ertapenem dosage and administration: Difference between revisions
Gerald Chi (talk | contribs) mNo edit summary |
Gerald Chi (talk | contribs) mNo edit summary |
||
| Line 4: | Line 4: | ||
==Dosage and Administration== | ==Dosage and Administration== | ||
====Instructions for Use in All Patients==== | ====Instructions for Use in All Patients==== | ||
DO NOT MIX OR CO-INFUSE INVANZ WITH OTHER MEDICATIONS. DO NOT USE DILUENTS CONTAINING DEXTROSE (α-D-GLUCOSE). | DO NOT MIX OR CO-INFUSE INVANZ WITH OTHER MEDICATIONS. DO NOT USE DILUENTS CONTAINING DEXTROSE (α-D-GLUCOSE). | ||
| Line 10: | Line 12: | ||
====Treatment Regimen==== | ====Treatment Regimen==== | ||
======13 years of age and older====== | ======13 years of age and older====== | ||
| Line 27: | Line 30: | ||
====Prophylactic Regimen in Adults==== | ====Prophylactic Regimen in Adults==== | ||
Table 2 presents prophylaxis guidelines for INVANZ. | Table 2 presents prophylaxis guidelines for INVANZ. | ||
| Line 36: | Line 40: | ||
====Patients with Renal Impairment==== | ====Patients with Renal Impairment==== | ||
INVANZ may be used for the treatment of infections in adult patients with renal impairment. In patients whose creatinine clearance is >30 mL/min/1.73 m2, no dosage adjustment is necessary. Adult patients with severe renal impairment (creatinine clearance ≤30 mL/min/1.73 m2) and end-stage renal disease (creatinine clearance ≤10 mL/min/1.73 m2) should receive 500 mg daily. A supplementary dose of 150 mg is recommended if ertapenem is administered within 6 hours prior to hemodialysis. There are no data in pediatric patients with renal impairment. | INVANZ may be used for the treatment of infections in adult patients with renal impairment. In patients whose creatinine clearance is >30 mL/min/1.73 m2, no dosage adjustment is necessary. Adult patients with severe renal impairment (creatinine clearance ≤30 mL/min/1.73 m2) and end-stage renal disease (creatinine clearance ≤10 mL/min/1.73 m2) should receive 500 mg daily. A supplementary dose of 150 mg is recommended if ertapenem is administered within 6 hours prior to hemodialysis. There are no data in pediatric patients with renal impairment. | ||
====Patients on Hemodialysis==== | ====Patients on Hemodialysis==== | ||
When adult patients on hemodialysis are given the recommended daily dose of 500 mg of INVANZ within 6 hours prior to hemodialysis, a supplementary dose of 150 mg is recommended following the hemodialysis session. If INVANZ is given at least 6 hours prior to hemodialysis, no supplementary dose is needed. There are no data in patients undergoing peritoneal dialysis or hemofiltration. There are no data in pediatric patients on hemodialysis. | When adult patients on hemodialysis are given the recommended daily dose of 500 mg of INVANZ within 6 hours prior to hemodialysis, a supplementary dose of 150 mg is recommended following the hemodialysis session. If INVANZ is given at least 6 hours prior to hemodialysis, no supplementary dose is needed. There are no data in patients undergoing peritoneal dialysis or hemofiltration. There are no data in pediatric patients on hemodialysis. | ||
| Line 48: | Line 54: | ||
====Patients with Hepatic Impairment==== | ====Patients with Hepatic Impairment==== | ||
No dose adjustment recommendations can be made in patients with hepatic impairment [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)]. | No dose adjustment recommendations can be made in patients with hepatic impairment [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)]. | ||
====Preparation and Reconstitution for Administration==== | ====Preparation and Reconstitution for Administration==== | ||
=====Adults and pediatric patients 13 years of age and older===== | =====Adults and pediatric patients 13 years of age and older===== | ||
======Preparation for intravenous administration====== | ======Preparation for intravenous administration====== | ||
DO NOT MIX OR CO-INFUSE INVANZ WITH OTHER MEDICATIONS. DO NOT USE DILUENTS CONTAINING DEXTROSE (α-D-GLUCOSE). | DO NOT MIX OR CO-INFUSE INVANZ WITH OTHER MEDICATIONS. DO NOT USE DILUENTS CONTAINING DEXTROSE (α-D-GLUCOSE). | ||
| Line 62: | Line 72: | ||
======Preparation for intramuscular administration====== | ======Preparation for intramuscular administration====== | ||
INVANZ MUST BE RECONSTITUTED PRIOR TO ADMINISTRATION. | INVANZ MUST BE RECONSTITUTED PRIOR TO ADMINISTRATION. | ||
| Line 71: | Line 82: | ||
======Preparation for Intravenous Administration====== | ======Preparation for Intravenous Administration====== | ||
DO NOT MIX OR CO-INFUSE INVANZ WITH OTHER MEDICATIONS. DO NOT USE DILUENTS CONTAINING DEXTROSE (α-D-GLUCOSE). | DO NOT MIX OR CO-INFUSE INVANZ WITH OTHER MEDICATIONS. DO NOT USE DILUENTS CONTAINING DEXTROSE (α-D-GLUCOSE). | ||
| Line 81: | Line 93: | ||
======Preparation for Intramuscular Administration====== | ======Preparation for Intramuscular Administration====== | ||
INVANZ MUST BE RECONSTITUTED PRIOR TO ADMINISTRATION. | INVANZ MUST BE RECONSTITUTED PRIOR TO ADMINISTRATION. | ||
Revision as of 22:22, 5 January 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Dosage and Administration
Instructions for Use in All Patients
DO NOT MIX OR CO-INFUSE INVANZ WITH OTHER MEDICATIONS. DO NOT USE DILUENTS CONTAINING DEXTROSE (α-D-GLUCOSE).
INVANZ may be administered by intravenous infusion for up to 14 days or intramuscular injection for up to 7 days. When administered intravenously, INVANZ should be infused over a period of 30 minutes. Intramuscular administration of INVANZ may be used as an alternative to intravenous administration in the treatment of those infections for which intramuscular therapy is appropriate.
Treatment Regimen
13 years of age and older
The dose of INVANZ in patients 13 years of age and older is 1 gram (g) given once a day [see Clinical Pharmacology (12.3)].
3 months to 12 years of age
The dose of INVANZ in patients 3 months to 12 years of age is 15 mg/kg twice daily (not to exceed 1 g/day).
Table 1 presents treatment guidelines for INVANZ.
 |
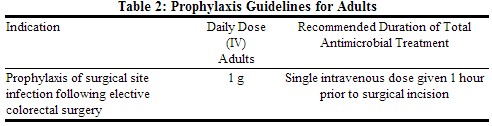
Prophylactic Regimen in Adults
Table 2 presents prophylaxis guidelines for INVANZ.
 |
Patients with Renal Impairment
INVANZ may be used for the treatment of infections in adult patients with renal impairment. In patients whose creatinine clearance is >30 mL/min/1.73 m2, no dosage adjustment is necessary. Adult patients with severe renal impairment (creatinine clearance ≤30 mL/min/1.73 m2) and end-stage renal disease (creatinine clearance ≤10 mL/min/1.73 m2) should receive 500 mg daily. A supplementary dose of 150 mg is recommended if ertapenem is administered within 6 hours prior to hemodialysis. There are no data in pediatric patients with renal impairment.
Patients on Hemodialysis
When adult patients on hemodialysis are given the recommended daily dose of 500 mg of INVANZ within 6 hours prior to hemodialysis, a supplementary dose of 150 mg is recommended following the hemodialysis session. If INVANZ is given at least 6 hours prior to hemodialysis, no supplementary dose is needed. There are no data in patients undergoing peritoneal dialysis or hemofiltration. There are no data in pediatric patients on hemodialysis.
When only the serum creatinine is available, the following formula may be used to estimate creatinine clearance. The serum creatinine should represent a steady state of renal function.
Males: (weight in kg) x (140-age in years) / (72) x serum creatinine (mg/100 mL)
Females: (0.85) x (value calculated for males)
Patients with Hepatic Impairment
No dose adjustment recommendations can be made in patients with hepatic impairment [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)].
Preparation and Reconstitution for Administration
Adults and pediatric patients 13 years of age and older
Preparation for intravenous administration
DO NOT MIX OR CO-INFUSE INVANZ WITH OTHER MEDICATIONS. DO NOT USE DILUENTS CONTAINING DEXTROSE (α-D-GLUCOSE).
INVANZ MUST BE RECONSTITUTED AND THEN DILUTED PRIOR TO ADMINISTRATION.
Reconstitute the contents of a 1 g vial of INVANZ with 10 mL of one of the following: Water for Injection, 0.9% Sodium Chloride Injection or Bacteriostatic Water for Injection. Shake well to dissolve and immediately transfer contents of the reconstituted vial to 50 mL of 0.9% Sodium Chloride Injection. Complete the infusion within 6 hours of reconstitution.
Preparation for intramuscular administration
INVANZ MUST BE RECONSTITUTED PRIOR TO ADMINISTRATION.
Reconstitute the contents of a 1 g vial of INVANZ with 3.2 mL of 1.0% lidocaine HCl injection2 (without epinephrine). Shake vial thoroughly to form solution. Immediately withdraw the contents of the vial and administer by deep intramuscular injection into a large muscle mass (such as the gluteal muscles or lateral part of the thigh). The reconstituted IM solution should be used within 1 hour after preparation. NOTE: THE RECONSTITUTED SOLUTION SHOULD NOT BE ADMINISTERED INTRAVENOUSLY.
Pediatric patients 3 months to 12 years of age
Preparation for Intravenous Administration
DO NOT MIX OR CO-INFUSE INVANZ WITH OTHER MEDICATIONS. DO NOT USE DILUENTS CONTAINING DEXTROSE (α-D-GLUCOSE).
INVANZ MUST BE RECONSTITUTED AND THEN DILUTED PRIOR TO ADMINISTRATION.
Reconstitute the contents of a 1 g vial of INVANZ with 10 mL of one of the following: Water for Injection, 0.9% Sodium Chloride Injection or Bacteriostatic Water for Injection. Shake well to dissolve and immediately withdraw a volume equal to 15 mg/kg of body weight (not to exceed 1 g/day) and dilute in 0.9% Sodium Chloride Injection to a final concentration of 20 mg/mL or less.
Complete the infusion within 6 hours of reconstitution.
Preparation for Intramuscular Administration
INVANZ MUST BE RECONSTITUTED PRIOR TO ADMINISTRATION.
Reconstitute the contents of a 1 g vial of INVANZ with 3.2 mL of 1.0% lidocaine HCl injection2 (without epinephrine). Shake vial thoroughly to form solution. Immediately withdraw a volume equal to 15 mg/kg of body weight (not to exceed 1 g/day) and administer by deep intramuscular injection into a large muscle mass (such as the gluteal muscles or lateral part of the thigh). The reconstituted IM solution should be used within 1 hour after preparation.
NOTE: THE RECONSTITUTED SOLUTION SHOULD NOT BE ADMINISTERED INTRAVENOUSLY.[1]
References
- ↑ "http://www.accessdata.fda.gov/drugsatfda_docs/label/2005/021337s018lbl.pdf" (PDF). External link in
|title=(help)
Adapted from the FDA Package Insert.