Ertapenem description
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
INVANZ (Ertapenem for Injection) is a sterile, synthetic, parenteral, 1-β methyl-carbapenem that is structurally related to beta-lactam antibiotics.
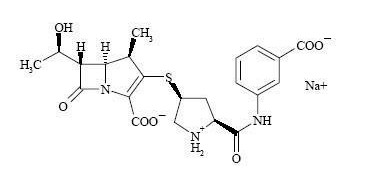
Chemically, INVANZ is described as [4R-[3(3S*,5S*),4α,5β,6β(R*)]]-3-[[5-[[(3-carboxyphenyl)amino]carbonyl]-3-pyrrolidinyl]thio]-6-(1-hydroxyethyl)-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid monosodium salt. Its molecular weight is 497.50. The empirical formula is C22H24N3O7SNa, and its structural formula is:
 |
Ertapenem sodium is a white to off-white hygroscopic, weakly crystalline powder. It is soluble in water and 0.9% sodium chloride solution, practically insoluble in ethanol, and insoluble in isopropyl acetate and tetrahydrofuran.
INVANZ is supplied as sterile lyophilized powder for intravenous infusion after reconstitution with appropriate diluent [see Dosage and Administration (2.7)] and transfer to 50 mL 0.9% Sodium Chloride Injection or for intramuscular injection following reconstitution with 1% lidocaine hydrochloride. Each vial contains 1.046 grams ertapenem sodium, equivalent to 1 gram ertapenem. The sodium content is approximately 137 mg (approximately 6.0 mEq).
Each vial of INVANZ contains the following inactive ingredients: 175 mg sodium bicarbonate and sodium hydroxide to adjust pH to 7.5.[1]
References
- ↑ "http://www.accessdata.fda.gov/drugsatfda_docs/label/2005/021337s018lbl.pdf" (PDF). External link in
|title=(help)
Adapted from the FDA Package Insert.