Ergotamine tartrate (sublingual tablet)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
Serious and/or life-threatening peripheral ischemia has been associated with the coadministration of ergotamine tartrate with potent CYP 3A4 inhibitors including protease inhibitors and macrolide antibiotics. Because CYP 3A4 inhibition elevates the serum levels of ergotamine tartrate, the risk for vasospasm leading to cerebral ischemia and/or ischemia of the extremities is increased. Hence, concomitant use of these medications is contraindicated.
|
Overview
Ergotamine tartrate (sublingual tablet) is an alpha adrenergic blocking agent that is FDA approved for the prophylaxis of vascular migraine. There is a Black Box Warning for this drug as shown here. Common adverse reactions include pruritus, nausea and vomiting, muscle weakness, paresthesia and visual disturbance.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Abortion or prevention of vascular headache, e.g., migraine, migraine variants or a so-called "histaminic cephalalgia"
- For best results, dosage should start at the first sign of an attack. Early administration gives maximum effectiveness. At the first sign of an attack or to relieve symptoms after onset of an attack, one 2 mg tablet is placed under the tongue. Another tablet should be taken at half-hour intervals thereafter, if necessary, but dosage must not exceed three tablets in any 24hour period. Total weekly dosage should not exceed five tablets (10 mg) in any one week. Sublingual Tablets should not be used for chronic daily administration.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ergotamine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ergotamine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Ergotamine tartrate (sublingual tablet) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ergotamine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ergotamine in pediatric patients.
Contraindications
- Coadministration of ergotamine with potent CYP 3A4 inhibitors (ritonavir, nelfinavir, indinavir, erythromycin, clarithromycin and troleandomycin) has been associated with acute ergot toxicity (ergotism) characterized by vasospasm and ischemia of the extremities, with some cases resulting in amputation. There have been rare reports of cerebral ischemia in patients on protease inhibitor therapy when ergotamine was coadministered, at least one resulting in death. Because of the increased risk for ergotism and other serious vasospastic adverse events, ergotamine use is contraindicated with these drugs and other potent inhibitors of CYP3A4 (e.g., ketoconazole, itraconazole).
- Ergomar Sublingual Tablets may cause fetal harm when administered to pregnant women. Ergomar Sublingual Tablets are contraindicated in women who are or may become pregnant. If this drug is used during pregnancy or if the patient becomes pregnant while taking this product, the patient should be apprised of the potential hazard to the fetus. Peripheral vascular disease, coronary heart disease, hypertension, impaired hepatic function or renal function and sepsisHypersensitivity to any of the components.
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
Serious and/or life-threatening peripheral ischemia has been associated with the coadministration of ergotamine tartrate with potent CYP 3A4 inhibitors including protease inhibitors and macrolide antibiotics. Because CYP 3A4 inhibition elevates the serum levels of ergotamine tartrate, the risk for vasospasm leading to cerebral ischemia and/or ischemia of the extremities is increased. Hence, concomitant use of these medications is contraindicated.
|
CYP 3A4 Inhibitors (e.g. Macrolide Antibiotics and Protease Inhibitors)
- Coadministration of ergotamine with potent CYP3A4 inhibitors such as protease inhibitors or macrolide antibiotics has been associated with serious adverse events; for this reason, these drugs should not be given concomitantly with ergotamine. While these reactions have not been reported with less potent CYP3A4 inhibitors, there is a potential risk for serious toxicity including vasospasm when these drugs are used with ergotamine. Examples of less potent CYP3A4 inhibitors include: saquinavir, nefazodone, fluconazole, fluoxetine, grapefruit juice, fluvoxamine, zileuton, metronidazole, and clotrimazole. These lists are not exhaustive, and the prescriber should consider the effects on CYP3A4 of other agents being considered for concomitant use with ergotamine.
Fibrotic Complications
- There have been a few reports of patients on ergotamine tartrate and caffeine therapy developing retroperitoneal and/or pleuropulmonary fibrosis. There have also been rare reports of fibrotic thickening of the aortic valve, mitral valve, tricuspid valve, and/or pulmonary valve with long-term continuous use of ergotamine tartrate and caffeine. Ergomar Sublingual Tablets should not be used for chronic daily administration
Adverse Reactions
Clinical Trials Experience
Cardiovascular Effects
Gastrointestinal Effects
Neurological Effects
Allergic Effects
Fibrotic Complications
Postmarketing Experience
There is limited information regarding Ergotamine tartrate (sublingual tablet) Postmarketing Experience in the drug label.
Drug Interactions
- Pharmacokinetic interactions (increased blood levels of ergotamine) have been reported in patients treated orally with ergotamine and macrolide antibiotics (e.g., troleandomycin, clarithromycin, erythromycin), and in patients treated orally with ergotamine and protease inhibitors (e.g. ritonavir) presumably due to inhibition of cytochrome P450 3A metabolism of ergotamine. Ergotamine has also been shown to be an inhibitor of cytochrome P450 3A catalyzed reactions. No pharmacokinetic interactions involving other cytochrome P450 isoenzymes are known.
Use in Specific Populations
Pregnancy
- There are no studies on the placental transfer or teratogenicity of Ergomar. Ergotamine crosses the placenta in small amounts, although it does not appear to be embryotoxic in this quantity. However, prolonged vasoconstriction of the uterine vessels and/or increased myometrial tone leading to reduced myometrial and placental blood flow may have contributed to fetal growth retardation observed in animals.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ergotamine tartrate (sublingual tablet) in women who are pregnant.
Labor and Delivery
- Ergomar is contraindicated in pregnancy due to its oxytocic effect which is maximal in the third trimester.
Nursing Mothers
- Ergot drugs are known to inhibit prolactin but there are no reports of decreased lactation with Ergomar. Ergotamine is excreted in breast milk and may cause symptoms of vomiting, diarrhea, weak pulse and unstable blood pressure in nursing infants. Because of the potential for serious adverse reactions in nursing infants from Ergomar, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
There is no FDA guidance on the use of Ergotamine tartrate (sublingual tablet) in geriatric settings.
Gender
There is no FDA guidance on the use of Ergotamine tartrate (sublingual tablet) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ergotamine tartrate (sublingual tablet) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Ergotamine tartrate (sublingual tablet) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Ergotamine tartrate (sublingual tablet) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Ergotamine tartrate (sublingual tablet) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Ergotamine tartrate (sublingual tablet) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Sublingual tablets
Monitoring
There is limited information regarding Ergotamine tartrate (sublingual tablet) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Ergotamine tartrate (sublingual tablet) and IV administrations.
Overdosage
- Symptoms include vomiting, numbness, tingling, pain and cyanosis of the extremities associated with diminished or absent peripheral pulses; hypertension or hypotension; drowsiness, stupor, coma, convulsions and shock. A case has been reported of reversible bilateral papillitis with ring scotomata in a patient who received five times the recommended daily adult dose over a period of 14 days. Treatment consists of removal of the offending drug. Maintenance of adequate pulmonary ventilation, correction of hypotension, and control of convulsions and blood pressure are important considerations. Treatment of peripheral vasospasm should consist of warmth, but not heat, and protection of the ischemic limbs. Vasodilators may be beneficial but caution must be exercised to avoid aggravating an already existent hypotension.
Pharmacology
Mechanism of Action
- Ergotamine is a alpha adrenergic blocking agent with a direct stimulating effect on the smooth muscle of peripheral and cranial blood vessels and produces depression of central vasomotor centers. The compound also has the properties of serotonin antagonism. In comparison to hydrogenated ergotamine, the adrenergic blocking actions are less pronounced and vasoconstrictive actions are greater.
Structure
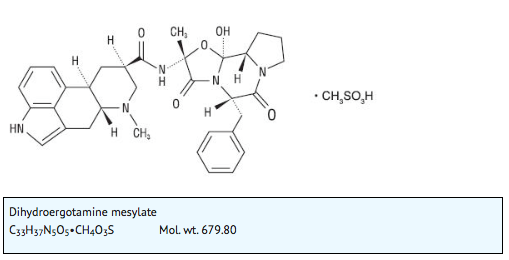
- D.H.E. 45® is ergotamine hydrogenated in the 9, 10 position as the mesylate salt. D.H.E. 45® is known chemically as ergotaman-3´,6´,18-trione,9,10-dihydro-12´-hydroxy-2´-methyl-5´-(phenylmethyl)-,(5´α)-, monomethanesulfonate. Its molecular weight is 679.80 and its empirical formula is C33H37N5O5•CH4O3S.
- The chemical structure is:
Pharmacodynamics
There is limited information regarding Ergotamine tartrate (sublingual tablet) Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Ergotamine tartrate (sublingual tablet) Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Ergotamine tartrate (sublingual tablet) Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Ergotamine tartrate (sublingual tablet) Clinical Studies in the drug label.
How Supplied
Ergotamine Tartrate Sublingual Tablets
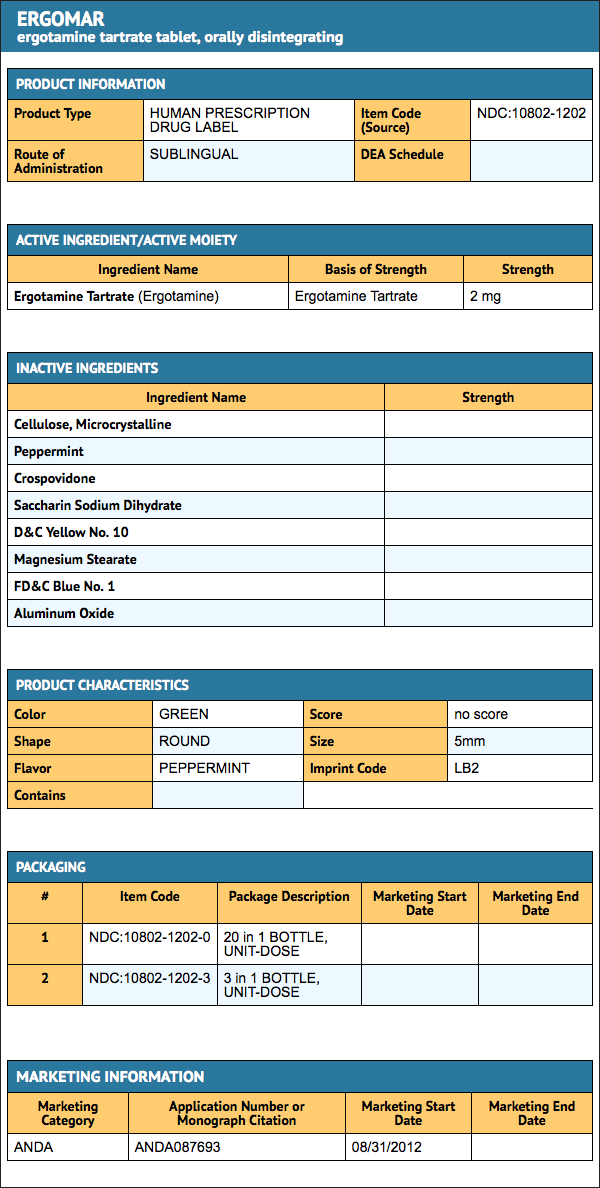
- Ergomar® Sublingual Tablets are round, green tablets each containing 2 mg of ergotamine tartrate. They are debossed with the product identification code "LB2" on one side, and are supplied in individual foil strips packaged in a plastic child resistant canister containing 20 tablets (5 – 2 × 2 foil strips) NDC 10802-12020
Storage
- Store at 20°- 25°C (68° - 77°F); excursions permitted to 15° - 30°C (59° - 86°F). Protect from light and heat. Keep out of reach of children.
Images
Drug Images
{{#ask: Page Name::Ergotamine tartrate (sublingual tablet) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Ergotamine tartrate (sublingual tablet) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Ergotamine tartrate (sublingual tablet) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Ergotamine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- [[]]
- Ergomar®[1]
Look-Alike Drug Names
There is limited information regarding Ergotamine tartrate (sublingual tablet) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Ergotamine tartrate (sublingual tablet) |Label Name=CP2.png
}}