Erenumab
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Yashasvi Aryaputra[2];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Erenumab is a calcitonin gene-related peptide receptor antagonist that is FDA approved for the preventive treatment of migraine in adults. Common adverse reactions include injection site reactions and constipation.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications:
- Erenumab is indicated for the preventive treatment of migraine in adults.
Recommended Dosing:
- The recommended dosage of Erenumab is 70 mg injected subcutaneously once monthly. Some patients may benefit from a dosage of 140 mg injected subcutaneously once monthly, which is administered as two consecutive subcutaneous injections of 70 mg each.
- If a dose of Erenumab is missed, administer as soon as possible. Thereafter, Erenumab can be scheduled monthly from the date of the last dose.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Erenumab Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding Erenumab Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Erenumab FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Erenumab Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding Erenumab Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
- None
Warnings
There is limited information regarding Erenumab Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
- The safety of Erenumab has been evaluated in 2,537 patients with migraine who received at least one dose of Erenumab, representing 2,310 patient-years of exposure. Of these, 2,057 patients were exposed to 70 mg or 140 mg once monthly for at least 6 months, 1,198 patients were exposed for at least 12 months, and 287 patients were exposed for at least 18 months.
- In placebo-controlled clinical studies (Studies 1, 2, and 3) of 2,184 patients, 787 patients received at least one dose of Erenumab 70 mg once monthly, 507 patients received at least one dose of Erenumab 140 mg once monthly, and 890 patients received placebo during 3 months or 6 months of double-blind treatment. Approximately 84% were female, 91% were white, and the mean age was 42 years at study entry.
- The most common adverse reactions (incidence ≥ 3% and more often than placebo) in the migraine studies were injection site reactions and constipation. Table 1 summarizes the adverse reactions that occurred during the first 3 months in the migraine studies (Studies 1, 2, and 3).
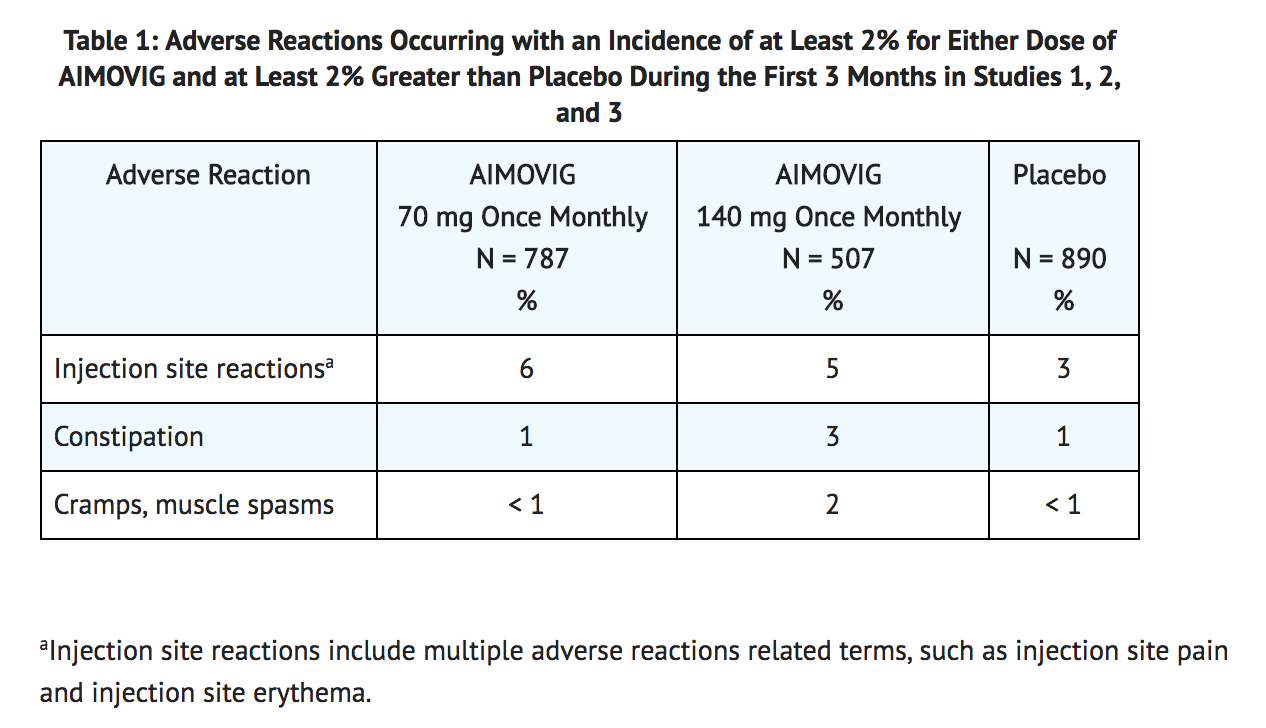
- In Studies 1, 2, and 3, 1.3% of patients treated with Erenumab discontinued double-blind treatment because of adverse events. The most frequent injection site reactions were injection site pain, injection site erythema, and injection site pruritus.
Immunogenicity
- As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation, including neutralizing antibodies, is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to Erenumab-aooe in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
- The immunogenicity of Erenumab has been evaluated using an immunoassay for the detection of binding anti-Erenumab-aooe antibodies. For patients whose sera tested positive in the screening immunoassay, an in vitro biological assay was performed to detect neutralizing antibodies.
- In controlled studies with Erenumab, the incidence of anti-Erenumab-aooe antibody development was 6.2% (48/778) in patients receiving Erenumab 70 mg once monthly (2 of whom had in vitro neutralizing activity) and 2.6% (13/504) in patients receiving Erenumab 140 mg once monthly (none of whom had in vitro neutralizing activity). The neutralizing anti-Erenumab-aooe antibody positive rate may be underestimated because of limitations of the assay. Although these data do not demonstrate an impact of anti-Erenumab-aooe antibody development on the efficacy or safety of Erenumab in these patients, the available data are too limited to make definitive conclusions.
Postmarketing Experience
There is limited information regarding Erenumab Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Erenumab Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Risk Summary
- There are no adequate data on the developmental risk associated with the use of Erenumab in pregnant women. No adverse effects on offspring were observed when pregnant monkeys were administered Erenumab-aooe throughout gestation. Serum Erenumab-aooe exposures in pregnant monkeys were greater than those in humans at clinical doses.
- In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2%-4% and 15%-20%, respectively. The estimated rate of major birth defects (2.2%-2.9%) and miscarriage (17%) among deliveries to women with migraine are similar to rates reported in women without migraine.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
- Published data have suggested that women with migraine may be at increased risk of preeclampsia during pregnancy.
Data (Animal)
- In a study in which female monkeys were administered Erenumab-aooe (0 or 50 mg/kg) twice weekly by subcutaneous injection throughout pregnancy (gestation day 20-22 to parturition), no adverse effects on offspring were observed. Serum Erenumab-aooe exposures (AUC) in pregnant monkeys were approximately 20 times that in humans at a dose of 140 mg once monthly.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Erenumab in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Erenumab during labor and delivery.
Nursing Mothers
Risk Summary
- There are no data on the presence of Erenumab-aooe in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Erenumab and any potential adverse effects on the breastfed infant from Erenumab or from the underlying maternal condition.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
- Clinical studies of Erenumab did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Erenumab with respect to specific gender populations.
Race
There is no FDA guidance on the use of Erenumab with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Erenumab in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Erenumab in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Erenumab in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Erenumab in patients who are immunocompromised.
Administration and Monitoring
Administration
- Erenumab is for subcutaneous use only.
- The needle shield within the white cap of the Erenumab prefilled autoinjector and gray needle cap of the Erenumab prefilled syringe contain dry natural rubber (a derivative of latex), which may cause allergic reactions in individuals sensitive to latex.
- Erenumab is intended for patient self-administration. Prior to use, provide proper training to patients and/or caregivers on how to prepare and administer Erenumab using the single-dose prefilled autoinjector or single-dose prefilled syringe, including aseptic technique.
- Prior to subcutaneous administration, allow Erenumab to sit at room temperature for at least 30 minutes protected from direct sunlight [see How Supplied/Storage and Handling (16.2)]. Do not warm by using a heat source such as hot water or a microwave.
- Do not shake the product.
- Inspect visually for particulate matter and discoloration prior to administration [see Dosage Forms and Strengths (3)]. Do not use if the solution is cloudy or discolored or contains flakes or particles.
- Administer Erenumab in the abdomen, thigh, or upper arm subcutaneously. Do not inject into areas where the skin is tender, bruised, red, or hard.
- Both prefilled autoinjector and prefilled syringe are single-dose and deliver the entire contents.
Monitoring
There is limited information regarding Erenumab Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Erenumab and IV administrations.
Overdosage
There is limited information regarding Erenumab overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Mechanism of Action
- Erenumab-aooe is a human monoclonal antibody that binds to the calcitonin gene-related peptide (CGRP) receptor and antagonizes CGRP receptor function.
Structure
There is limited information regarding Erenumab Structure in the drug label.
Pharmacodynamics
- In a randomized, double-blind, placebo-controlled study in healthy volunteers, concomitant administration of Erenumab-aooe (140 mg intravenous, single-dose) with sumatriptan (12 mg subcutaneous, given as two 6 mg doses separated by one hour) had no effect on resting blood pressure compared with sumatriptan alone. Erenumab is for subcutaneous use only.
Pharmacokinetics
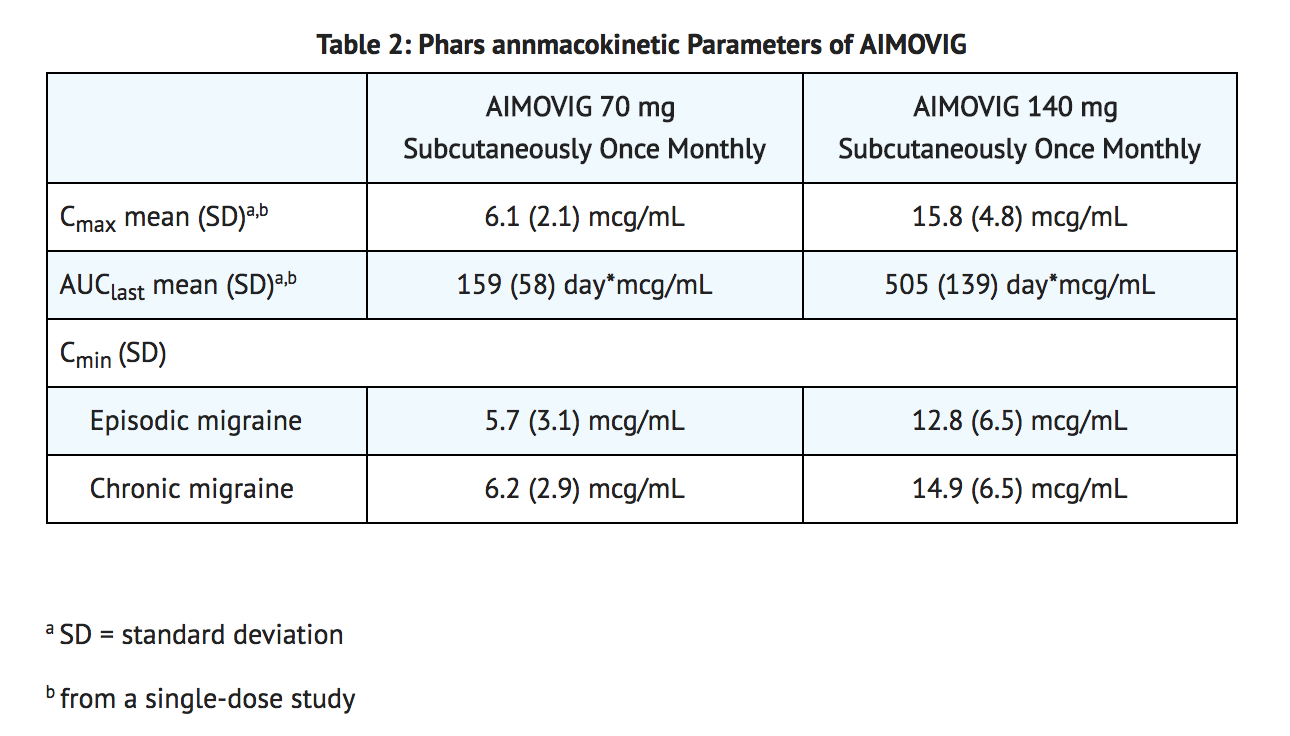
- Erenumab-aooe exhibits non-linear kinetics as a result of binding to the CGRP receptor. The Cmax mean and AUClast mean following subcutaneous administration of a 70 mg once monthly and a 140 mg once monthly dose in healthy volunteers or migraine patients are included in Table 2.
- Less than 2-fold accumulation was observed in trough serum concentrations (Cmin) for episodic and chronic migraine patients following subcutaneous administration of 70 mg once monthly and 140 mg once monthly doses (see Table 2). Serum trough concentrations approached steady state by 3 months of dosing. The effective half-life of Erenumab-aooe is 28 days.
Absorption
- Following a single subcutaneous dose of 70 mg or 140 mg Erenumab-aooe administered to healthy adults, median peak serum concentrations were attained in approximately 6 days, and estimated absolute bioavailability was 82%.
Distribution
- Following a single 140 mg intravenous dose, the mean (SD) volume of distribution during the terminal phase (Vz) was estimated to be 3.86 (0.77) L.
Metabolism and Excretion
- Two elimination phases were observed for Erenumab-aooe. At low concentrations, the elimination is predominantly through saturable binding to target (CGRP receptor), while at higher concentrations the elimination of Erenumab-aooe is largely through a non-specific, non-saturable proteolytic pathway.
Specific Populations
- The pharmacokinetics of Erenumab-aooe were not affected by age, gender, race, or subtypes of migraine spectrum (episodic or chronic migraine) based on population pharmacokinetics analysis.
Patients with Renal or Hepatic Impairment
- Population pharmacokinetic analysis of integrated data from the Erenumab clinical studies did not reveal a difference in the pharmacokinetics of Erenumab-aooe in patients with mild or moderate renal impairment relative to those with normal renal function. Patients with severe renal impairment (eGFR < 30 mL/min/1.73 m2) have not been studied. No dedicated clinical studies were conducted to evaluate the effect of hepatic impairment or renal impairment on the pharmacokinetics of Erenumab-aooe. Renal or hepatic impairment is not expected to affect pharmacokinetics of Erenumab-aooe.
Drug Interaction Studies
P450 Enzymes
- Erenumab-aooe is not metabolized by cytochrome P450 enzymes; therefore, interactions with concomitant medications that are substrates, inducers, or inhibitors of cytochrome P450 enzymes are unlikely.
Oral Contraceptives
- In an open-label drug interaction study in healthy female volunteers, Erenumab-aooe (140 mg subcutaneous, single-dose) did not affect the pharmacokinetics of a combined oral contraceptive containing ethinyl estradiol and norgestimate.
Sumatriptan
- In a study in healthy volunteers, concomitant administration of Erenumab-aooe with sumatriptan had no effect on the pharmacokinetics of sumatriptan.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
- The carcinogenic potential of Erenumab-aooe has not been assessed.
Mutagenesis
- Genetic toxicology studies of Erenumab-aooe have not been conducted.
Impairment of Fertility
- Mating studies have not been conducted on Erenumab-aooe. No histopathological changes in male or female reproductive organs were observed in monkeys administered Erenumab-aooe (0, 25, or 150 mg/kg) by subcutaneous injection twice weekly for up to 6 months. Serum Erenumab-aooe exposures (AUC) at the higher dose tested were more than 100 times that in humans at a dose of 140 mg once monthly.
Clinical Studies
- The efficacy of Erenumab was evaluated as a preventive treatment of episodic or chronic migraine in three randomized, double-blind, placebo-controlled studies: two studies in patients with episodic migraine (4 to 14 migraine days per month) (Study 1 and Study 2) and one study in patients with chronic migraine (≥ 15 headache days per month with ≥ 8 migraine days per month) (Study 3). The studies enrolled patients with a history of migraine, with or without aura, according to the International Classification of Headache Disorders (ICHD-III) diagnostic criteria.
Episodic Migraine
- Study 1 (NCT 02456740) was a randomized, multi-center, 6-month, placebo-controlled, double-blind study evaluating Erenumab for the preventive treatment of episodic migraine. A total of 955 patients with a history of episodic migraine were randomized to receive either Erenumab 70 mg (N = 317), Erenumab 140 mg (N = 319), or placebo (N = 319) by subcutaneous injection once monthly (QM) for 6 months. Patients were allowed to use acute headache treatments including migraine-specific medications (i.e., triptans, ergotamine derivatives) and NSAIDs during the study.
- The study excluded patients with medication overuse headache as well as patients with myocardial infarction, stroke, transient ischemic attacks, unstable angina, coronary artery bypass surgery, or other revascularization procedures within 12 months prior to screening.
- The primary efficacy endpoint was the change from baseline in mean monthly migraine days over months 4 to 6. Secondary endpoints included the achievement of a ≥ 50% reduction from baseline in mean monthly migraine days over months 4 to 6 (“≥ 50% MMD responders”), the change from baseline in mean monthly acute migraine-specific medication days over months 4 to 6, and the change from baseline in mean Migraine Physical Function Impact Diary (MPFID) over months 4 to 6. The MPFID measures the impact of migraine on everyday activities (EA) and physical impairment (PI) using an electronic diary administered daily. Monthly MPFID scores are averaged over 28 days, including days with and without migraine; scores are scaled from 0 to 100. Higher scores indicate worse impact on EA and PI. Reductions from baseline in MPFID scores indicate improvement.
- A total of 858 (90%) patients completed the 6-month double-blind study. Patients had a median age of 42 years (range: 18 to 65 years), 85% were female, and 89% were white. Three percent of patients were taking concomitant preventive treatments for migraine. The mean migraine frequency at baseline was approximately 8 migraine days per month and was similar across treatment groups.
- Erenumab treatment demonstrated statistically significant improvements for key efficacy endpoints compared to placebo, as summarized in Table 3.
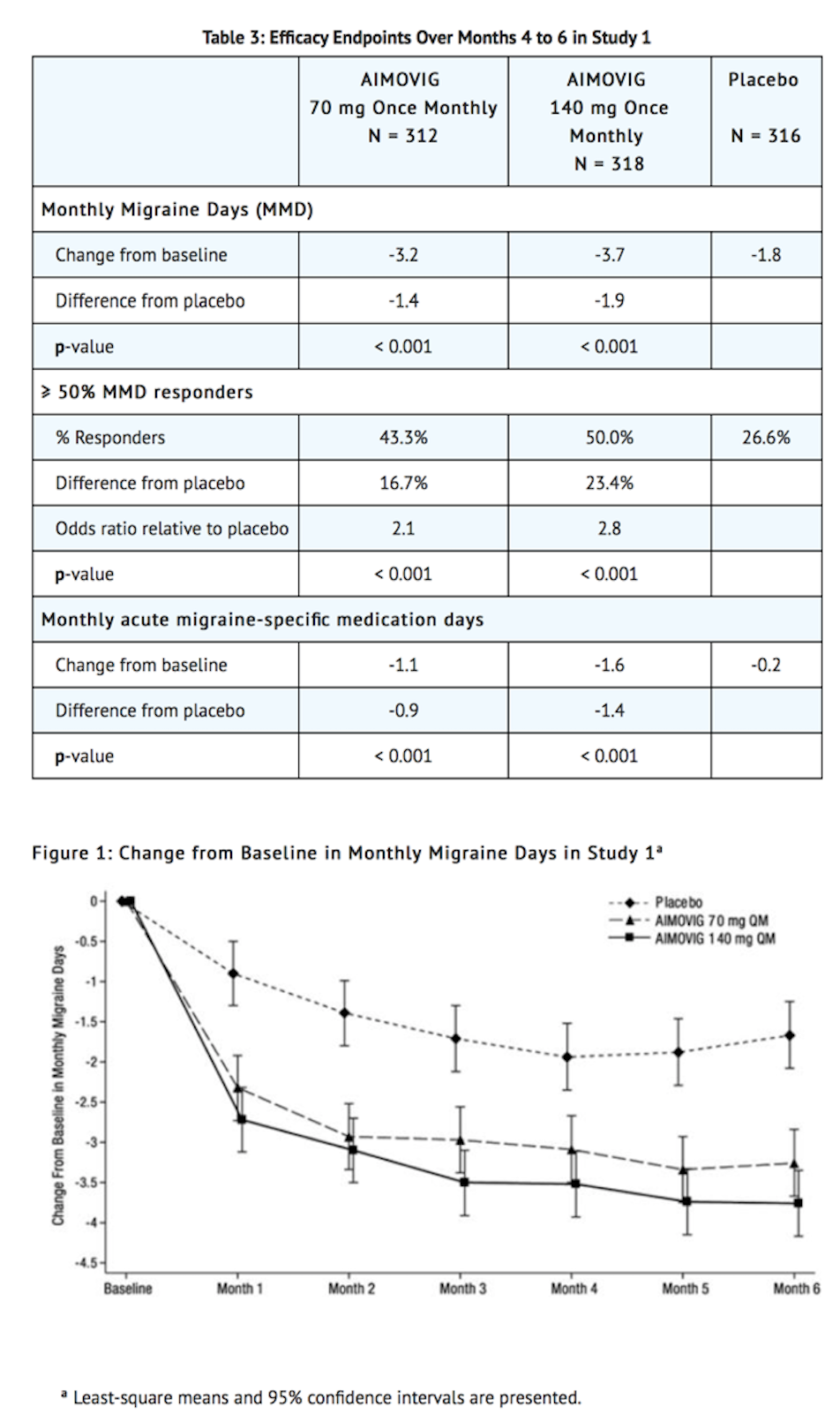
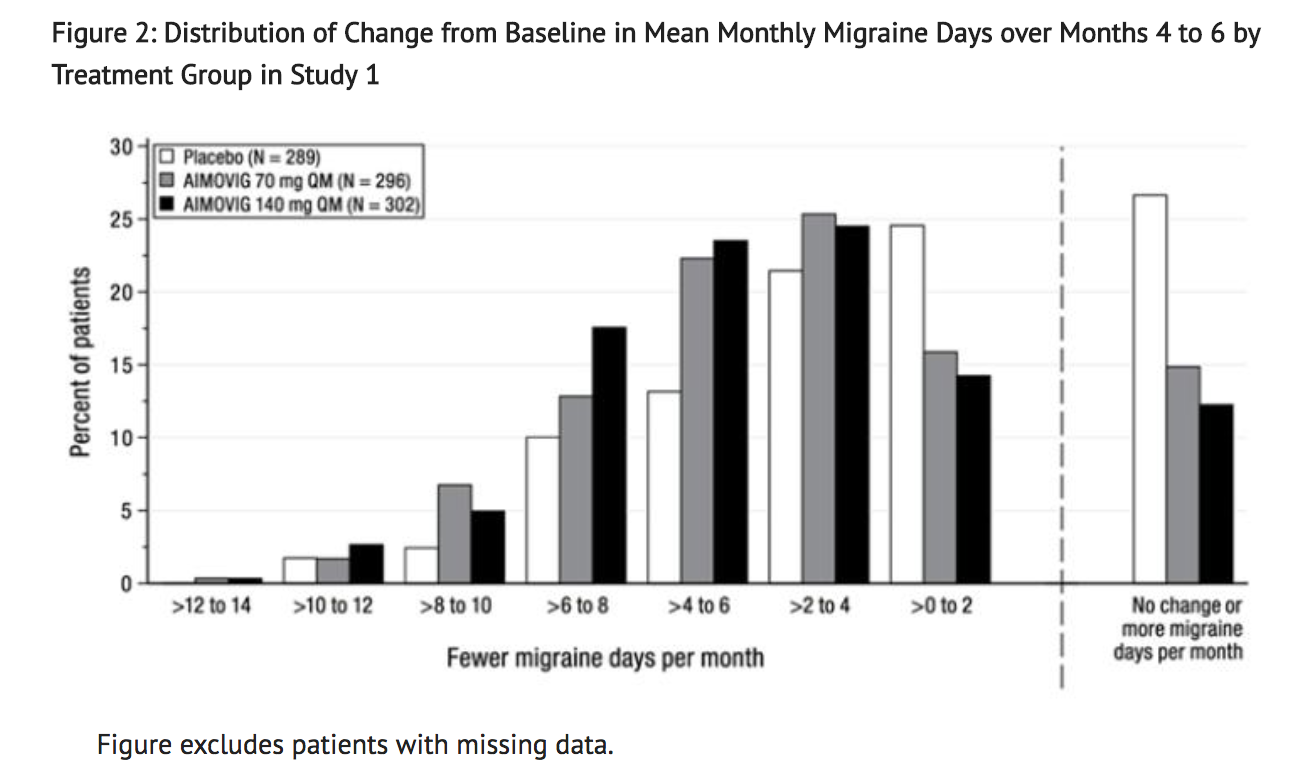
- Figure 2 shows the distribution of change from baseline in mean monthly migraine days over months 4 to 6 in bins of 2 days by treatment group. A treatment benefit over placebo for both doses of Erenumab is seen across a range of changes from baseline in monthly migraine days.
- Compared to placebo, patients treated with Erenumab 70 mg once monthly and 140 mg once monthly showed greater reductions from baseline in mean monthly MPFID everyday activity scores averaged over months 4 to 6 [difference from placebo: -2.2 for Erenumab 70 mg and -2.6 for Erenumab 140 mg; p-value < 0.001 for both], and in mean monthly MPFID physical impairment scores averaged over months 4 to 6 [difference from placebo: -1.9 for Erenumab 70 mg and -2.4 for Erenumab 140 mg; p-value < 0.001 for both].
- Study 2 (NCT 02483585) was a randomized, multi-center, 3-month, placebo-controlled, double-blind study evaluating Erenumab for the preventive treatment of episodic migraine. A total of 577 patients with a history of episodic migraine were randomized to receive either Erenumab 70 mg (N = 286) or placebo (N = 291) by subcutaneous injection once monthly for 3 months. Patients were allowed to use acute headache treatments including migraine-specific medications (i.e., triptans, ergotamine derivatives) and NSAIDs during the study.
- The study excluded patients with medication overuse headache as well as patients with myocardial infarction, stroke, transient ischemic attacks, unstable angina, coronary artery bypass surgery, or other revascularization procedures within 12 months prior to screening.
- The primary efficacy endpoint was the change from baseline in monthly migraine days at month 3. Secondary endpoints included the achievement of a ≥ 50% reduction from baseline in monthly migraine days (“≥ 50% MMD responders”), the change from baseline in monthly acute migraine-specific medication days at month 3, and the proportion of patients with at least a 5-point score reduction from baseline in MPFID at month 3.
- A total of 546 (95%) patients completed the 3-month double-blind study. Patients had a median age of 43 years (range: 18 to 65 years), 85% were female, and 90% were white. Six to seven percent of patients were taking concomitant preventive migraine treatment. The mean migraine frequency at baseline was approximately 8 migraine days per month and was similar between treatment groups.
- Erenumab treatment demonstrated statistically significant improvements for key efficacy endpoints compared to placebo, as summarized in Table 4.
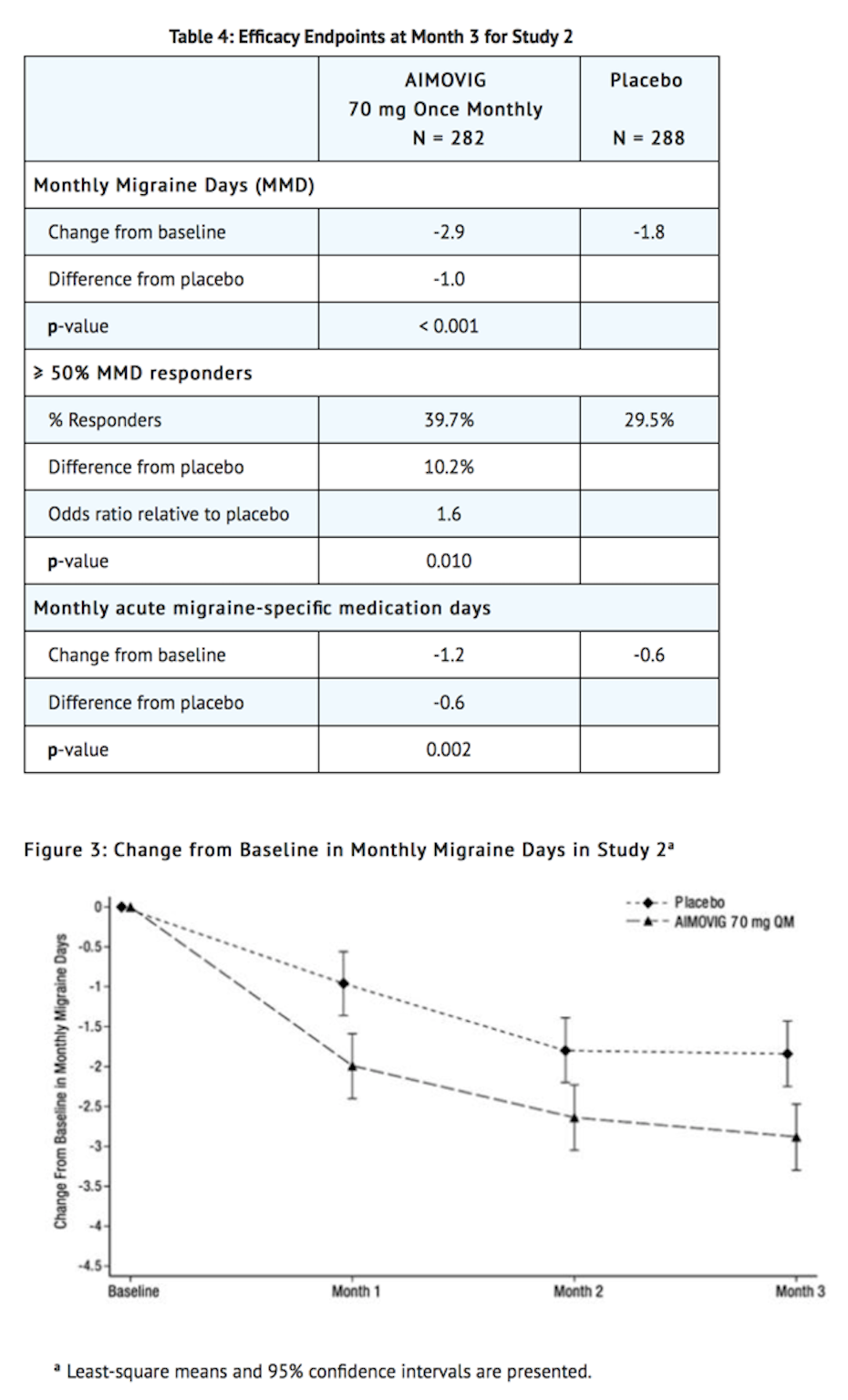
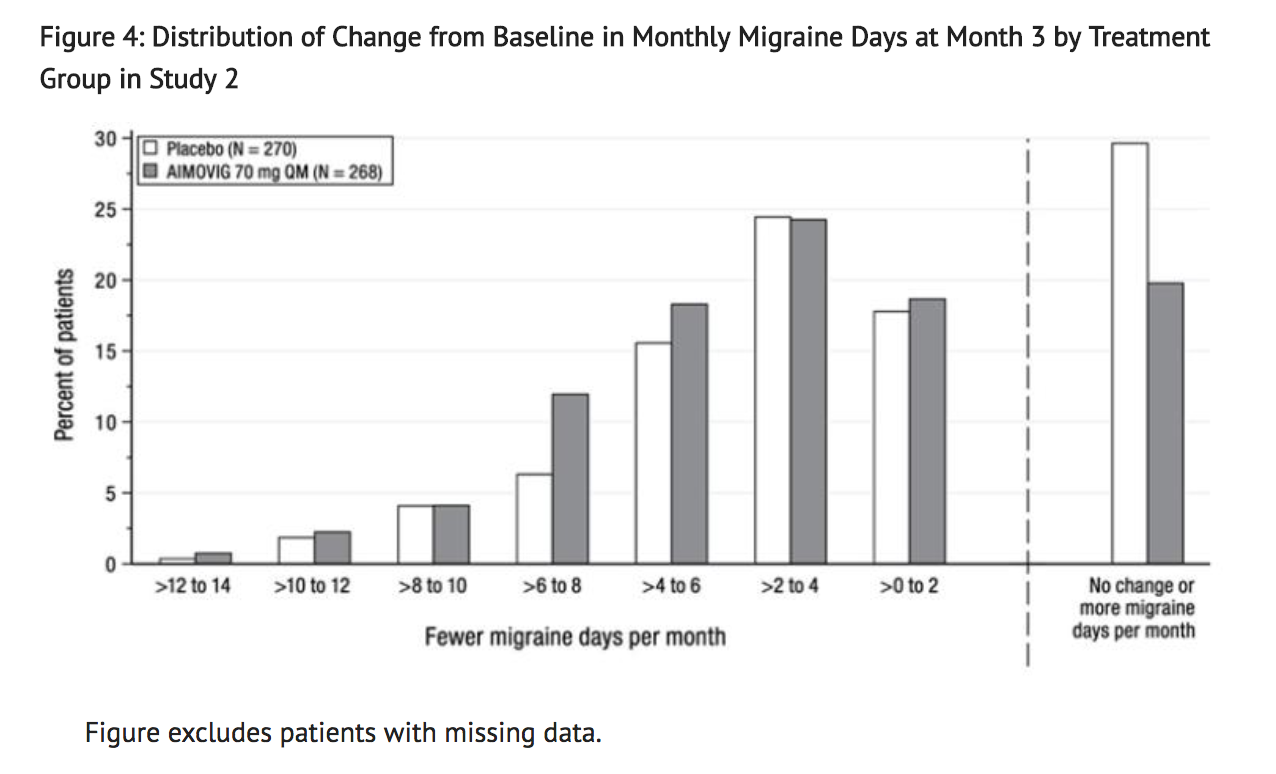
- Figure 4 shows the distribution of change from baseline in monthly migraine days at month 3 in bins of 2 days by treatment group. A treatment benefit over placebo for Erenumab is seen across a range of changes from baseline in monthly migraine days.
- The pre-specified analysis for the MPFID was based on at least a 5-point reduction within-patient responder definition. Erenumab 70 mg once monthly was not significantly better than placebo for the proportion of responders for everyday activity [difference from placebo: 4.7%; odds ratio = 1.2; p-value = 0.26] and physical impairment [difference from placebo: 5.9%; odds ratio = 1.3; p-value = 0.13]. In an exploratory analysis of the change from baseline in the mean MPFID scores at month 3, patients treated with Erenumab 70 mg, as compared to placebo, showed nominally greater reductions of physical impairment scores [difference from placebo: -1.3; p-value = 0.021], but not of everyday activities scores [difference from placebo: -1.1; p-value = 0.061].
Chronic Migraine
- Study 3 (NCT 02066415) was a randomized, multi-center, 3-month, placebo-controlled, double-blind study evaluating Erenumab as a preventive treatment of chronic migraine. A total of 667 patients with a history of chronic migraine with or without aura were randomized to receive Erenumab 70 mg (N = 191), Erenumab 140 mg (N = 190), or placebo (N = 286) by subcutaneous injections once monthly for 3 months. Patients were allowed to use acute headache treatments including migraine-specific medications (i.e., triptans, ergotamine derivatives) and NSAIDs during the study.
- The study excluded patients with medication overuse headache caused by opiate overuse and patients with concurrent use of migraine preventive treatments. Patients with myocardial infarction, stroke, transient ischemic attacks, unstable angina, coronary artery bypass surgery, or other revascularization procedures within 12 months prior to screening were also excluded.
- The primary efficacy endpoint was the change from baseline in monthly migraine days at month 3. Secondary endpoints included the achievement of a ≥ 50% reduction from baseline in monthly migraine days (“≥ 50% MMD responders”) and change from baseline in monthly acute migraine-specific medication days at month 3.
- A total of 631 (95%) patients completed the 3-month double-blind study. Patients had a median age of 43 years (range: 18 to 66 years), 83% were female, and 94% were white. The mean migraine frequency at baseline was approximately 18 migraine days per month and was similar across treatment groups.
- Erenumab treatment demonstrated statistically significant improvements for key efficacy outcomes compared to placebo, as summarized in Table 5.
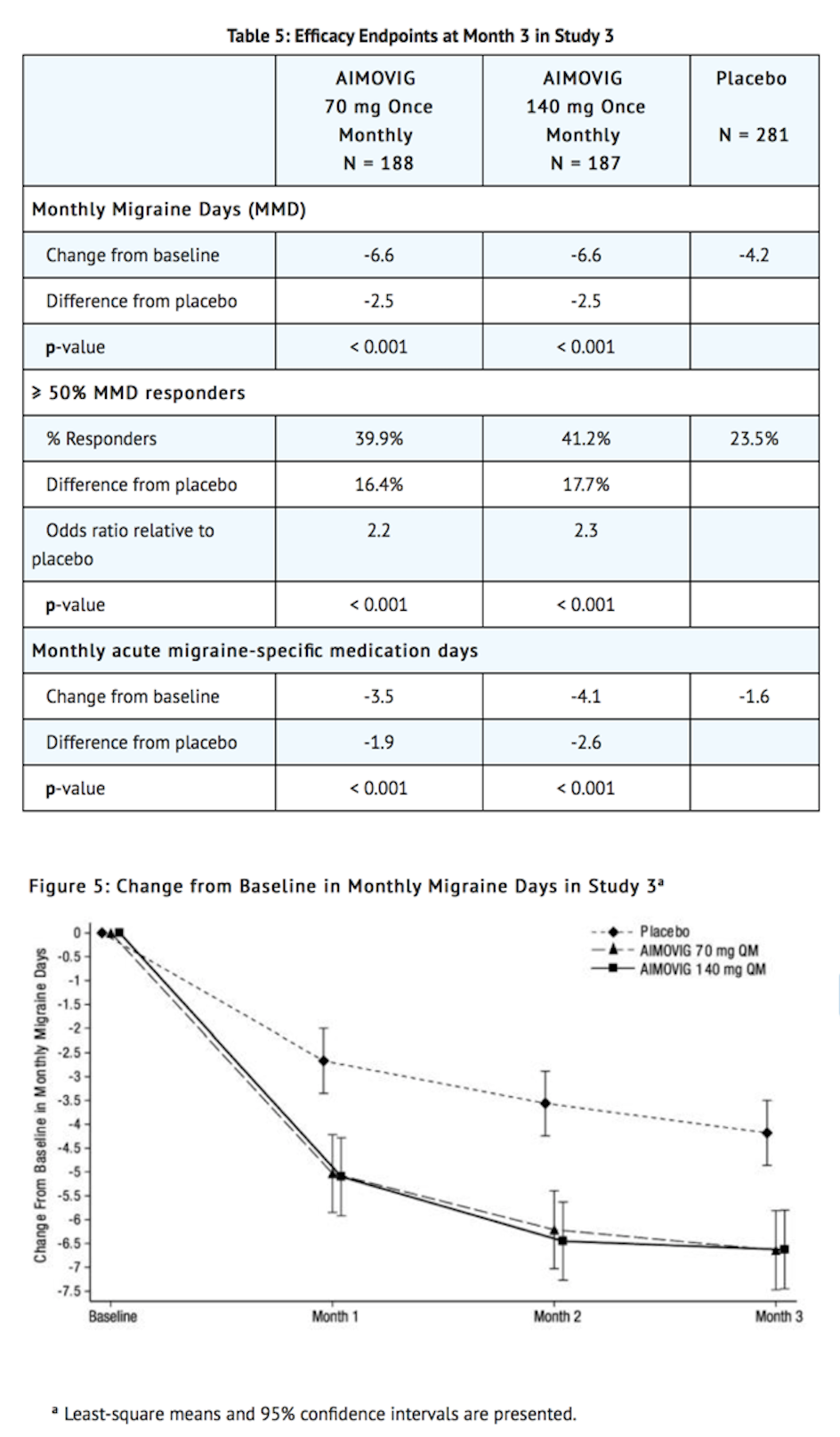
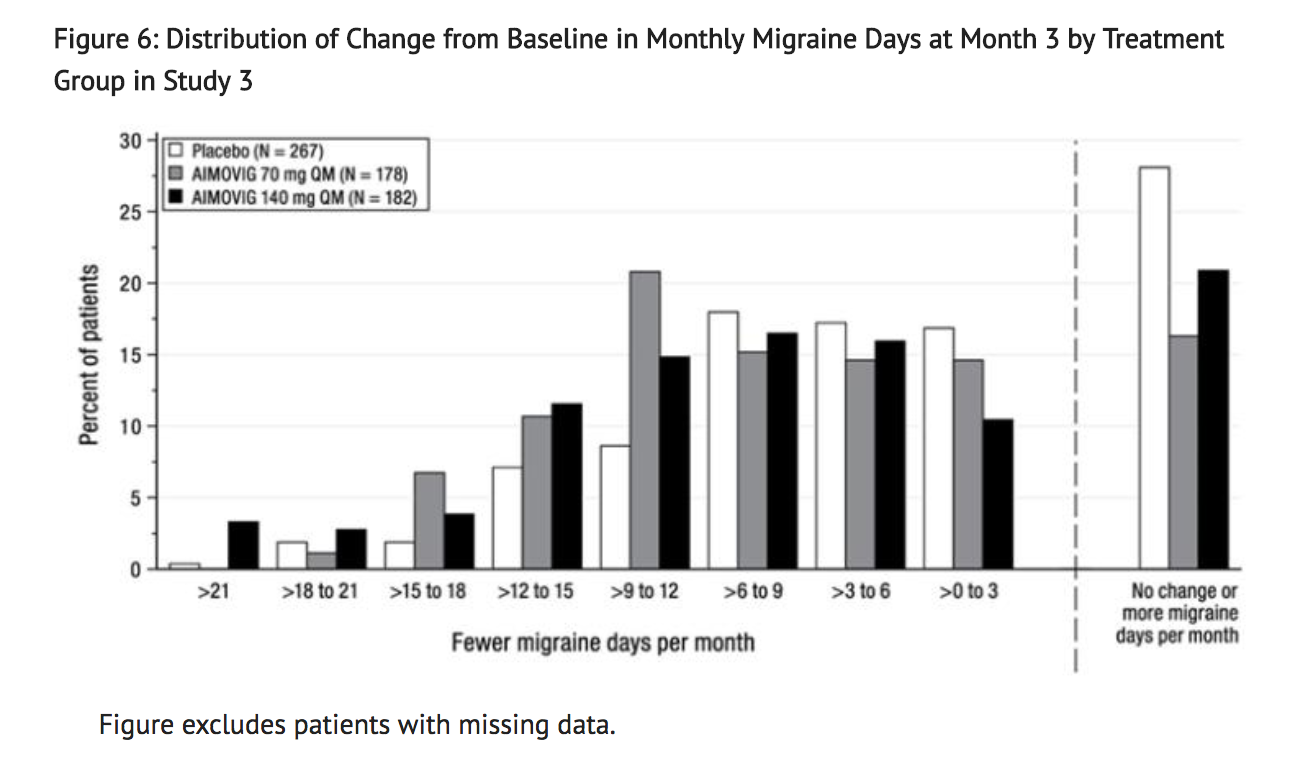
- Figure 6 shows the distribution of change from baseline in monthly migraine days at month 3 in bins of 3 days by treatment group. A treatment benefit over placebo for both doses of Erenumab is seen across a range of changes from baseline in migraine days.
How Supplied
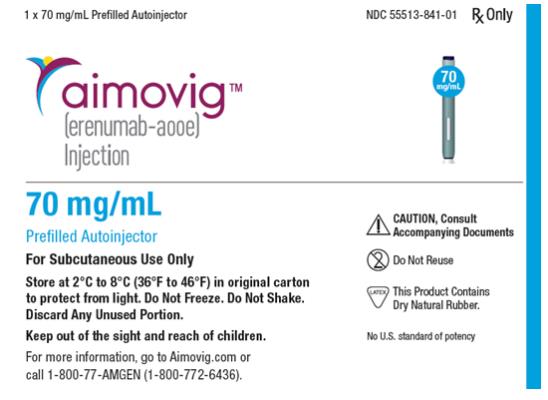
- Erenumab (Erenumab-aooe) injection is a sterile, clear to opalescent, colorless to light yellow solution for subcutaneous administration.
- The needle shield within the white cap of the Erenumab prefilled autoinjector and gray needle cap of the Erenumab prefilled syringe contain dry natural rubber (a derivative of latex). Each single-dose prefilled SureClick® autoinjector or single-dose prefilled syringe of Erenumab contains a Type 1 glass syringe and stainless steel needle and delivers 1 mL of 70 mg/mL solution.
- Erenumab is supplied as follows:
SureClick® Autoinjector
- Pack of 1 autoinjector: 70 mg/mL single-dose prefilled autoinjector NDC 55513-841-01.
- Pack of 2 autoinjectors: 140 mg/2 mL (2 x 70 mg/mL single-dose prefilled autoinjectors) NDC 55513-841-02
Syringe
- Pack of 1 syringe: 70 mg/mL single-dose prefilled syringe NDC 55513-840-01
- Pack of 2 syringes: 140 mg/2 mL (2 x 70 mg/mL single-dose prefilled syringes) NDC 55513-840-02
Storage
- Store refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light until time of use.
- If removed from the refrigerator, Erenumab should be kept at room temperature (up to 25°C [77°F]) in the original carton and must be used within 7 days. Throw away Erenumab that has been left at room temperature for more than 7 days.
- Do not freeze.
- Do not shake.
Images
Drug Images
{{#ask: Page Name::Erenumab |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Erenumab |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Information on Preparation and Administration:
- Provide guidance to patients and caregivers on proper subcutaneous administration technique, including aseptic technique, and how to use the single-dose prefilled autoinjector or single-dose prefilled syringe. Instruct patients and/or caregivers to read and follow the Instructions for Use each time they use Erenumab.
- Instruct patients prescribed 140 mg to administer the once monthly dosage as two separate subcutaneous injections of 70 mg each.
- Advise latex-sensitive patients that the needle shield within the white cap of the Erenumab prefilled autoinjector and gray needle cap of the Erenumab prefilled syringe contain dry natural rubber (a derivative of latex) that may cause allergic reactions in individuals sensitive to latex.

Precautions with Alcohol
Alcohol-Erenumab interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Aimovig
Look-Alike Drug Names
There is limited information regarding Erenumab Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.