Enalapril labels and packages: Difference between revisions
Jump to navigation
Jump to search
Amr Marawan (talk | contribs) (Created page with "__NOTOC__ {{Enalapril}} {{CMG}}; {{AE}} {{AM}} <ref name="dailymed.nlm.nih.gov">{{Cite web | last = | first = | title = VASOTEC (ENALAPRIL MALEATE) TABLET VASOTEC (ENAL...") |
Amr Marawan (talk | contribs) No edit summary |
||
| Line 3: | Line 3: | ||
{{CMG}}; {{AE}} {{AM}} | {{CMG}}; {{AE}} {{AM}} | ||
{| | |||
| [[File:012.png|800px|thumb]] | |||
|} | |||
{| | |||
| [[File:013.png|800px|thumb]] | |||
<ref name="dailymed.nlm.nih.gov">{{Cite web | last = | first = | title = VASOTEC (ENALAPRIL MALEATE) TABLET VASOTEC (ENALAPRIL MALEATE) TABLET [BTA PHARMACEUTICALS INC.] | url = http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=5d181743-a0fc-4d17-953d-b17a936f9ecc | publisher = | date = | accessdate = }}</ref> | |}<ref name="dailymed.nlm.nih.gov">{{Cite web | last = | first = | title = VASOTEC (ENALAPRIL MALEATE) TABLET VASOTEC (ENALAPRIL MALEATE) TABLET [BTA PHARMACEUTICALS INC.] | url = http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=5d181743-a0fc-4d17-953d-b17a936f9ecc | publisher = | date = | accessdate = }}</ref> | ||
==References== | ==References== | ||
Revision as of 21:31, 12 February 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [2], Amr Marawan, M.D. [3]
Enalapril
Enalapril and Hydrochlorothiazide
Enalapril and Felodipine
Overview
Enalapril tablet is an angiontensin converting enzyme inhibitor drug that is FDA approved for the treatment of hypertension, heart failure, left ventricular dysfunction after myocardial infarction, diabetic nephropathy. Adverse reactions include hypotension, rash, hyperkalemia, disorder of taste, cough. hypotension, rash, hyperkalemia, disorder of taste, cough.
Category
Antihypertensive Agents, Angiotensin Converting Enzyme Inhibitors. Editor-In-Chief: C. Michael Gibson, M.S., M.D. [4]; Associate Editor(s)-in-Chief: Amr Marawan, M.D. [5]
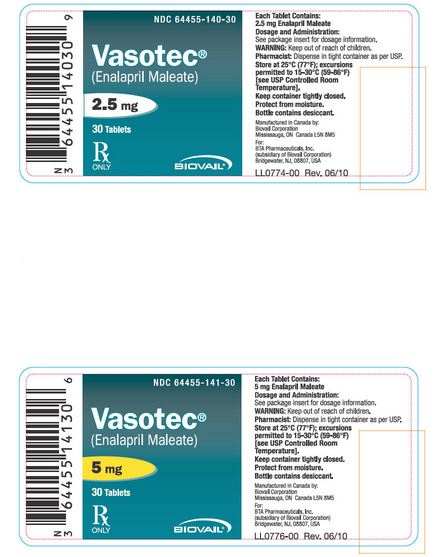 |
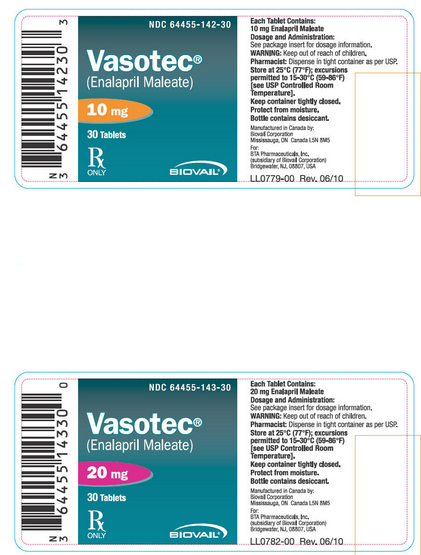 |
References
Adapted from the FDA Package Insert.