Empagliflozin and metformin hydrochloride
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Shivani Chaparala M.B.B.S [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
LACTIC ACIDOSIS:
|
Overview
Empagliflozin and metformin hydrochloride is a combination of empagliflozin, a sodium-glucose co-transporter 2 (SGLT2) inhibitor and metformin hydrochloride, a biguanide that is FDA approved for the treatment of in adults with type 2 diabetes mellitus when treatment with both empagliflozin and metformin hydrochloride is appropriate, as an adjunct to diet and exercise to improve glycemic control. Empagliflozin is indicated to reduce the risk of cardiovascular death in adults with type 2 diabetes mellitus and established cardiovascular disease. However, the effectiveness of SYNJARDY on reducing the risk of cardiovascular death in adults with type 2 diabetes mellitus and cardiovascular disease has not been established.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include * Most common adverse reactions associated with empagliflozin (5% or greater incidence) were urinary tract infection and female genital mycotic infections.
- Most common adverse reactions associated with metformin (>5%) are diarrhea, nausea/vomiting, flatulence, abdominal discomfort, indigestion, asthenia, and headache..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- SYNJARDY is a combination of empagliflozin, a sodium-glucose co-transporter 2 (SGLT2) inhibitor and metformin hydrochloride, a biguanide, indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus when treatment with both empagliflozin and metformin hydrochloride is appropriate.
- Empagliflozin is indicated to reduce the risk of cardiovascular death in adults with type 2 diabetes mellitus and established cardiovascular disease.
- However, the effectiveness of SYNJARDY on reducing the risk of cardiovascular death in adults with type 2 diabetes mellitus and cardiovascular disease has not been established.
DOSAGE FORMS AND STRENGTHS
- Tablets:
5 mg empagliflozin/500 mg metformin hydrochloride 5 mg empagliflozin/1000 mg metformin hydrochloride 12.5 mg empagliflozin/500 mg metformin hydrochloride 12.5 mg empagliflozin/1000 mg metformin hydrochloride.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Empagliflozin and metformin hydrochloride in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Empagliflozin and metformin hydrochloride in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Empagliflozin and metformin hydrochloride FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Empagliflozin and metformin hydrochloride in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Empagliflozin and metformin hydrochloride in pediatric patients.
Contraindications
- SYNJARDY is contraindicated in patients with:
- Moderate to severe renal impairment (eGFR less than 45 mL/min/1.73 m2), end stage renal disease, or dialysis.
- Acute or chronic metabolic acidosis, including diabetic ketoacidosis.
- Diabetic ketoacidosis should be treated with insulin.
- History of serious hypersensitivity reaction to empagliflozin or metformin.
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
LACTIC ACIDOSIS:
|
Lactic Acidosis
- There have been postmarketing cases of metformin-associated lactic acidosis, including fatal cases.
- These cases had a subtle onset and were accompanied by nonspecific symptoms such as malaise, myalgias, abdominal pain, respiratory distress, or increased somnolence; however, hypothermia, hypotension, and resistant bradyarrhythmias have occurred with severe acidosis.
- Metformin-associated lactic acidosis was characterized by elevated blood lactate concentrations (>5 mmol/Liter), anion gap acidosis (without evidence of ketonuria or ketonemia), and an increased lactate:pyruvate ratio; metformin plasma levels generally >5 mcg/mL.
- Metformin decreases liver uptake of lactate increasing lactate blood levels which may increase the risk of lactic acidosis, especially in patients at risk.
- If metformin-associated lactic acidosis is suspected, general supportive measures should be instituted promptly in a hospital setting, along with immediate discontinuation of SYNJARDY.
- In SYNJARDY-treated patients with a diagnosis or strong suspicion of lactic acidosis, prompt hemodialysis is recommended to correct the acidosis and remove accumulated metformin (metformin hydrochloride is dialyzable, with a clearance of up to 170 mL/minute under good hemodynamic conditions). * Hemodialysis has often resulted in reversal of symptoms and recovery.
- Educate patients and their families about the symptoms of lactic acidosis and if these symptoms occur instruct them to discontinue SYNJARDY and report these symptoms to their healthcare provider.
- For each of the known and possible risk factors for metformin-associated lactic acidosis, recommendations to reduce the risk of and manage metformin-associated lactic acidosis are provided below:
- Renal Impairment: The postmarketing metformin-associated lactic acidosis cases primarily occurred in patients with significant renal impairment.
- The risk of metformin accumulation and metformin-associated lactic acidosis increases with the severity of renal impairment because metformin is substantially excreted by the kidney.
- Before initiating SYNJARDY, obtain an estimated glomerular filtration rate (eGFR).
- SYNJARDY is contraindicated in patients with an eGFR below 45 mL/min/1.73 m2.
- Obtain an eGFR at least annually in all patients taking SYNJARDY. In patients at increased risk for the development of renal impairment (e.g., the elderly), renal function should be assessed more frequently.
Drug Interactions:
- The concomitant use of SYNJARDY with specific drugs may increase the risk of metformin-associated lactic acidosis: those that impair renal function, result in significant hemodynamic change, interfere with acid-base balance or increase metformin accumulation.
- Therefore, consider more frequent monitoring of patients.
Age 65 or Greater:
- The risk of metformin-associated lactic acidosis increases with the patient’s age because elderly patients have a greater likelihood of having hepatic, renal, or cardiac impairment than younger patients.
- Assess renal function more frequently in elderly patients.
Radiological Studies with Contrast:
- Administration of intravascular iodinated contrast agents in metformin-treated patients has led to an acute decrease in renal function and the occurrence of lactic acidosis.
- Stop SYNJARDY at the time of, or prior to, an iodinated contrast imaging procedure in patients with an eGFR between 45 and 60 mL/min/1.73 m2; in patients with a history of hepatic impairment, alcoholism, or heart failure; or in patients who will be administered intra-arterial iodinated contrast.
- Re-evaluate eGFR 48 hours after the imaging procedure, and restart SYNJARDY if renal function is stable.
Surgery and Other Procedures:
- Withholding of food and fluids during surgical or other procedures may increase the risk for volume depletion, hypotension and renal impairment.
- SYNJARDY should be temporarily discontinued while patients have restricted food and fluid intake.
Hypoxic States:
- Several of the postmarketing cases of metformin-associated lactic acidosis occurred in the setting of acute congestive heart failure (particularly when accompanied by hypoperfusion and hypoxemia).
- Cardiovascular collapse (shock), acute myocardial infarction, sepsis, and other conditions associated with hypoxemia have been associated with lactic acidosis and may also cause prerenal azotemia.
- When such events occur, discontinue SYNJARDY.
Excessive Alcohol Intake:
- Alcohol potentiates the effect of metformin on lactate metabolism and this may increase the risk of metformin-associated lactic acidosis.
- Warn patients against excessive alcohol intake while receiving SYNJARDY.
Hepatic Impairment:
- Patients with hepatic impairment have developed cases of metformin-associated lactic acidosis.
- This may be due to impaired lactate clearance resulting in higher lactate blood levels.
- Therefore, avoid use of SYNJARDY in patients with clinical or laboratory evidence of hepatic disease.
Hypotension
- Empagliflozin causes intravascular volume contraction.
- Symptomatic hypotension may occur after initiating empagliflozin particularly in patients with renal impairment, the elderly, in patients with low systolic blood pressure, and in patients on diuretics.
- Before initiating SYNJARDY, assess for volume contraction and correct volume status if indicated.
- Monitor for signs and symptoms of hypotension after initiating therapy and increase monitoring in clinical situations where volume contraction is expected.
Ketoacidosis
- Reports of ketoacidosis, a serious life-threatening condition requiring urgent hospitalization have been identified in postmarketing surveillance in patients with type 1 and type 2 diabetes mellitus receiving sodium glucose co-transporter-2 (SGLT2) inhibitors, including empagliflozin.
- Fatal cases of ketoacidosis have been reported in patients taking empagliflozin.
- SYNJARDY is not indicated for the treatment of patients with type 1 diabetes mellitus.
- Patients treated with SYNJARDY who present with signs and symptoms consistent with severe metabolic acidosis should be assessed for ketoacidosis regardless of presenting blood glucose levels, as ketoacidosis associated with SYNJARDY may be present even if blood glucose levels are less than 250 mg/dL.
- If ketoacidosis is suspected, SYNJARDY should be discontinued, patient should be evaluated, and prompt treatment should be instituted.
- Treatment of ketoacidosis may require insulin, fluid and carbohydrate replacement.
- In many of the postmarketing reports, and particularly in patients with type 1 diabetes, the presence of ketoacidosis was not immediately recognized and institution of treatment was delayed because presenting blood glucose levels were below those typically expected for diabetic ketoacidosis (often less than 250 mg/dL).
- Signs and symptoms at presentation were consistent with dehydration and severe metabolic acidosis and included nausea, vomiting, abdominal pain, generalized malaise, and shortness of breath.
- In some but not all cases, factors predisposing to ketoacidosis such as insulin dose reduction, acute febrile illness, reduced caloric intake due to illness or surgery, pancreatic disorders suggesting insulin deficiency (e.g., type 1 diabetes, history of pancreatitis or pancreatic surgery), and alcohol abuse were identified.
- Before initiating SYNJARDY, consider factors in the patient history that may predispose to ketoacidosis including pancreatic insulin deficiency from any cause, caloric restriction, and alcohol abuse.
- In patients treated with SYNJARDY consider monitoring for ketoacidosis and temporarily discontinuing SYNJARDY in clinical situations known to predispose to ketoacidosis (e.g., prolonged fasting due to acute illness or surgery).
Acute Kidney Injury and Impairment in Renal Function
- Empagliflozin causes intravascular volume contraction.
- There have been postmarketing reports of acute kidney injury, some requiring hospitalization and dialysis, in patients receiving SGLT2 inhibitors, including empagliflozin; some reports involved patients younger than 65 years of age.
- Before initiating SYNJARDY, consider factors that may predispose patients to acute kidney injury including hypovolemia, chronic renal insufficiency, congestive heart failure and concomitant medications (diuretics, ACE inhibitors, ARBs, NSAIDs).
- Consider temporarily discontinuing SYNJARDY in any setting of reduced oral intake (such as acute illness or fasting) or fluid losses (such as gastrointestinal illness or excessive heat exposure); monitor patients for signs and symptoms of acute kidney injury.
- If acute kidney injury occurs, discontinue SYNJARDY promptly and institute treatment.
- Empagliflozin increases serum creatinine and decreases eGFR.
- Patients with hypovolemia may be more susceptible to these changes.
- Renal function abnormalities can occur after initiating SYNJARDY.
- Renal function should be evaluated prior to initiation of SYNJARDY and monitored periodically thereafter.
- More frequent renal function monitoring is recommended in patients with an eGFR below 60 mL/min/1.73 m2.
- Use of SYNJARDY is contraindicated in patients with an eGFR less than 45 mL/min/1.73 m2.
Urosepsis and Pyelonephritis
- There have been postmarketing reports of serious urinary tract infections including urosepsis and pyelonephritis requiring hospitalization in patients receiving SGLT2 inhibitors, including empagliflozin.
- Treatment with SGLT2 inhibitors increases the risk for urinary tract infections.
- Evaluate patients for signs and symptoms of urinary tract infections and treat promptly, if indicated.
Hypoglycemia with Concomitant Use with Insulin and Insulin Secretagogues
Empagliflozin
- Insulin and insulin secretagogues are known to cause hypoglycemia.
- The risk of hypoglycemia is increased when empagliflozin is used in combination with insulin secretagogues (e.g., sulfonylurea) or insulin.
- Therefore, a lower dose of the insulin secretagogue or insulin may be required to reduce the risk of hypoglycemia when used in combination with SYNJARDY.
Metformin
- Hypoglycemia does not occur in patients receiving metformin alone under usual circumstances of use, but could occur when caloric intake is deficient, when strenuous exercise is not compensated by caloric supplementation, or during concomitant use with other glucose-lowering agents (such as SUs and insulin) or ethanol.
- Elderly, debilitated, or malnourished patients, and those with adrenal or pituitary insufficiency or alcohol intoxication are particularly susceptible to hypoglycemic effects.
- Hypoglycemia may be difficult to recognize in the elderly, and in people who are taking β-adrenergic blocking drugs.
- Monitor for a need to lower the dose of SYNJARDY to minimize the risk of hypoglycemia in these patients.
Genital Mycotic Infections
- Empagliflozin increases the risk for genital mycotic infections.
- Patients with a history of chronic or recurrent genital mycotic infections were more likely to develop mycotic genital infections.
- Monitor and treat as appropriate.
Vitamin B12 Levels
- In controlled, 29-week clinical trials of metformin, a decrease to subnormal levels of previously normal serum vitamin B12 levels, without clinical manifestations, was observed in approximately 7% of metformin-treated patients.
- Such decrease, possibly due to interference with B12 absorption from the B12-intrinsic factor complex, is, however, very rarely associated with anemia or neurologic manifestations due to the short duration (<1 year) of the clinical trials.
- This risk may be more relevant to patients receiving long-term treatment with metformin, and adverse hematologic and neurologic reactions have been reported postmarketing.
- The decrease in vitamin B12 levels appears to be rapidly reversible with discontinuation of metformin or vitamin B12 supplementation.
- Measurement of hematologic parameters on an annual basis is advised in patients on SYNJARDY and any apparent abnormalities should be appropriately investigated and managed.
- Certain individuals (those with inadequate vitamin B12 or calcium intake or absorption) appear to be predisposed to developing subnormal vitamin B12 levels.
- In these patients, routine serum vitamin B12 measurement at 2- to 3-year intervals may be useful.
Increased Low-Density Lipoprotein Cholesterol (LDL-C)
- Increases in LDL-C can occur with empagliflozin.
- Monitor and treat as appropriate.
Macrovascular Outcomes
- There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with SYNJARDY.
Adverse Reactions
Clinical Trials Experience
The following are the important adverse reactions:
- Lactic Acidosis
- Hypotension
- Ketoacidosis
- Acute Kidney Injury and Impairment in Renal Function
- Urosepsis and Pyelonephritis
- Hypoglycemia with Concomitant Use with Insulin and Insulin Secretagogues
- Genital Mycotic Infections
- Vitamin B12 Deficiency
- Increased Low-Density Lipoprotein Cholesterol (LDL-C).
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The safety of concomitantly administered empagliflozin (daily dose 10 mg and 25 mg) and metformin hydrochloride (mean daily dose of approximately 1800 mg) has been evaluated in 3456 patients with type 2 diabetes mellitus treated for 16 to 24 weeks, of which 926 patients received placebo, 1271 patients received a daily dose of empagliflozin 10 mg, and 1259 patients received a daily dose of empagliflozin 25 mg.
- Discontinuation of therapy due to adverse events across treatment groups was 3.0%, 2.8%, and 2.9% for placebo, empagliflozin 10 mg, and empagliflozin 25 mg, respectively.
Empagliflozin Add-On Combination Therapy with Metformin
- In a 24-week placebo-controlled trial of empagliflozin 10 mg and 25 mg administered once daily added to metformin, there were no adverse reactions reported regardless of investigator assessment of causality in ≥5% of patients and more commonly than in patients given placebo.
Empagliflozin Add-On Combination Therapy with Metformin and Sulfonylurea
- In a 24-week placebo-controlled trial of empagliflozin 10 mg and 25 mg administered once daily added to metformin and sulfonylurea, adverse reactions reported regardless of investigator assessment of causality in ≥5% of patients and more commonly than in patients given placebo are presented in Table 1.
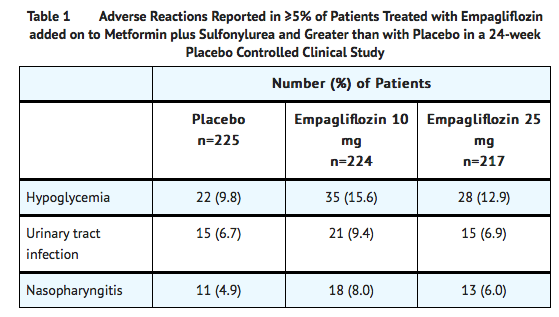
Empagliflozin
- The data in Table 2 are derived from a pool of four 24-week placebo-controlled trials and 18-week data from a placebo-controlled trial with basal insulin.
- Empagliflozin was used as monotherapy in one trial and as add-on therapy in four trials.
- These data reflect exposure of 1976 patients to empagliflozin with a mean exposure duration of approximately 23 weeks.
- Patients received placebo (N=995), empagliflozin 10 mg (N=999), or empagliflozin 25 mg (N=977) once daily.
- The mean age of the population was 56 years and 3% were older than 75 years of age.
- More than half (55%) of the population was male; 46% were White, 50% were Asian, and 3% were Black or African American.
- At baseline, 57% of the population had diabetes more than 5 years and had a mean hemoglobin A1c (HbA1c) of 8%.
- Established microvascular complications of diabetes at baseline included diabetic nephropathy (7%), retinopathy (8%), or neuropathy (16%).
- Baseline renal function was normal or mildly impaired in 91% of patients and moderately impaired in 9% of patients (mean eGFR 86.8 mL/min/1.73 m2).
- Table 2 shows common adverse reactions (excluding hypoglycemia) associated with the use of empagliflozin.
- The adverse reactions were not present at baseline, occurred more commonly on empagliflozin than on placebo and occurred in greater than or equal to 2% of patients treated with empagliflozin 10 mg or empagliflozin 25 mg.
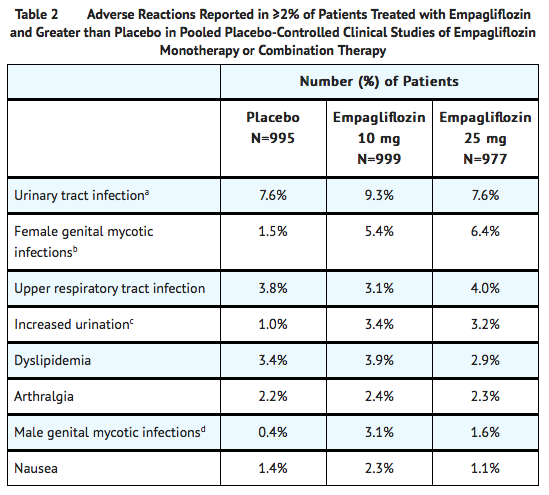
Volume Depletion
- Empagliflozin causes an osmotic diuresis, which may lead to intravascular volume contraction and adverse reactions related to volume depletion.
- In the pool of five placebo-controlled clinical trials, adverse reactions related to volume depletion (e.g., blood pressure (ambulatory) decreased, blood pressure systolic decreased, dehydration, hypotension, hypovolemia, orthostatic hypotension, and syncope) were reported by 0.3%, 0.5%, and 0.3% of patients treated with placebo, empagliflozin 10 mg, and empagliflozin 25 mg, respectively.
- Empagliflozin may increase the risk of hypotension in patients at risk for volume contraction.
Increased Urination
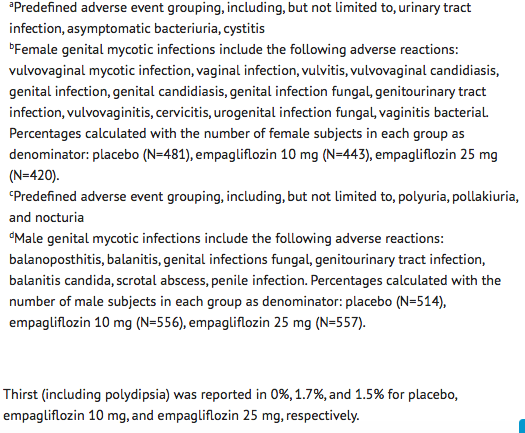
- In the pool of five placebo-controlled clinical trials, adverse reactions of increased urination (e.g., polyuria, pollakiuria, and nocturia) occurred more frequently on empagliflozin than on placebo (see Table 3).
- Specifically, nocturia was reported by 0.4%, 0.3%, and 0.8% of patients treated with placebo, empagliflozin 10 mg, and empagliflozin 25 mg, respectively.
Acute Impairment in Renal Function
- Treatment with empagliflozin was associated with increases in serum creatinine and decreases in eGFR.
- Patients with moderate renal impairment at baseline had larger mean changes.
- In a long-term cardiovascular outcome trial, the acute impairment in renal function was observed to reverse after treatment discontinuation suggesting acute hemodynamic changes play a role in the renal function changes observed with empagliflozin.
Hypoglycemia
- The incidence of hypoglycemia increased when empagliflozin was administered with insulin or sulfonylurea.
Genital Mycotic Infections
- In the pool of five placebo-controlled clinical trials, the incidence of genital mycotic infections (e.g., vaginal mycotic infection, vaginal infection, genital infection fungal, vulvovaginal candidiasis, and vulvitis) was increased in patients treated with empagliflozin compared to placebo, occurring in 0.9%, 4.1%, and 3.7% of patients randomized to placebo, empagliflozin 10 mg, and empagliflozin 25 mg, respectively.
- Discontinuation from study due to genital infection occurred in 0% of placebo-treated patients and 0.2% of patients treated with either empagliflozin 10 or 25 mg.
- Genital mycotic infections occurred more frequently in female than male patients.
- Phimosis occurred more frequently in male patients treated with empagliflozin 10 mg (less than 0.1%) and empagliflozin 25 mg (0.1%) than placebo (0%).
Urinary Tract Infections
- In the pool of five placebo-controlled clinical trials, the incidence of urinary tract infections (e.g., urinary tract infection, asymptomatic bacteriuria, and cystitis) was increased in patients treated with empagliflozin compared to placebo.
- Patients with a history of chronic or recurrent urinary tract infections were more likely to experience a urinary tract infection.
- The rate of treatment discontinuation due to urinary tract infections was 0.1%, 0.2%, and 0.1% for placebo, empagliflozin 10 mg, and empagliflozin 25 mg, respectively.
- Urinary tract infections occurred more frequently in female patients.
- The incidence of urinary tract infections in female patients randomized to placebo, empagliflozin 10 mg, and empagliflozin 25 mg was 16.6%, 18.4%, and 17.0%, respectively.
- The incidence of urinary tract infections in male patients randomized to placebo, empagliflozin 10 mg, and empagliflozin 25 mg was 3.2%, 3.6%, and 4.1%, respectively.
Metformin
- The most common (>5%) established adverse reactions due to initiation of metformin therapy are diarrhea, nausea/vomiting, flatulence, abdominal discomfort, indigestion, asthenia, and headache.
- Long-term treatment with metformin has been associated with a decrease in vitamin B12 absorption which may very rarely result in clinically significant vitamin B12 deficiency (e.g., megaloblastic anemia).
Laboratory Tests
Empagliflozin
- Increase in Low-Density Lipoprotein Cholesterol (LDL-C): Dose-related increases in low-density lipoprotein cholesterol (LDL-C) were observed in patients treated with empagliflozin.
- LDL-C increased by 2.3%, 4.6%, and 6.5% in patients treated with placebo, empagliflozin 10 mg, and empagliflozin 25 mg, respectively.
- The range of mean baseline LDL-C levels was 90.3 to 90.6 mg/dL across treatment groups.
- Increase in Hematocrit:
- In a pool of four placebo-controlled studies, median hematocrit decreased by 1.3% in placebo and increased by 2.8% in empagliflozin 10 mg and 2.8% in empagliflozin 25 mg treated patients.
- At the end of treatment, 0.6%, 2.7%, and 3.5% of patients with hematocrits initially within the reference range had values above the upper limit of the reference range with placebo, empagliflozin 10 mg, and empagliflozin 25 mg, respectively.
Metformin
- In controlled clinical trials of metformin of 29 weeks’ duration, a decrease to subnormal levels of previously normal serum Vitamin B12 levels, without clinical manifestations, was observed in approximately 7% of patients.
- Such decrease, possibly due to interference with B12 absorption from the B12-intrinsic factor complex, is, however, very rarely associated with anemia and appears to be rapidly reversible with discontinuation of metformin or Vitamin B12 supplementation.
Postmarketing Experience
- Additional adverse reactions have been identified during postapproval use of empagliflozin.
- Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Ketoacidosis
- Urosepsis and pyelonephritis.
Drug Interactions
Drug Interactions with Empagliflozin
- Diuretics:
- Coadministration of empagliflozin with diuretics resulted in increased urine volume and frequency of voids, which might enhance the potential for volume depletion.
- Insulin or Insulin Secretagogues:
- Coadministration of empagliflozin with insulin or insulin secretagogues increases the risk for hypoglycemia.
- Positive Urine Glucose Test:
- Monitoring glycemic control with urine glucose tests is not recommended in patients taking SGLT2 inhibitors as SGLT2 inhibitors increase urinary glucose excretion and will lead to positive urine glucose tests. ** Use alternative methods to monitor glycemic control.
- Interference with 1,5-anhydroglucitol (1,5-AG) Assay :
- Monitoring glycemic control with 1,5-AG assay is not recommended as measurements of 1,5-AG are unreliable in assessing glycemic control in patients taking SGLT2 inhibitors.
- Use alternative methods to monitor glycemic control.
Drug Interactions with Metformin Hydrochloride
- Drugs that Reduce Metformin Clearance:
- Concomitant use of drugs that interfere with common renal tubular transport systems involved in the renal elimination of metformin (e.g., organic cationic transporter-2 [OCT2] / multidrug and toxin extrusion [MATE] inhibitors such as ranolazine, vandetanib, dolutegravir, and cimetidine) could increase systemic exposure to metformin and may increase the risk for lactic acidosis. Consider the benefits and risks of concomitant use.
- Carbonic Anhydrase Inhibitors :
- Topiramate or other carbonic anhydrase inhibitors (e.g., zonisamide, acetazolamide or dichlorphenamide) frequently causes a decrease in serum bicarbonate and induce non-anion gap, hyperchloremic metabolic acidosis.
- Concomitant use of these drugs with SYNJARDY may increase the risk of lactic acidosis.
- Consider more frequent monitoring of these patients.
- Drugs Affecting Glycemic Control:
- Certain drugs tend to produce hyperglycemia and may lead to loss of glycemic control.
- These drugs include the thiazides and other diuretics, corticosteroids, phenothiazines, thyroid products, estrogens, oral contraceptives, phenytoin, nicotinic acid, sympathomimetics, calcium channel blocking drugs, and isoniazid.
- When such drugs are administered to a patient receiving SYNJARDY, the patient should be closely observed to maintain adequate glycemic control.
- When such drugs are withdrawn from a patient receiving SYNJARDY, the patient should be observed closely for hypoglycemia.
- Alcohol:
- Alcohol is known to potentiate the effect of metformin on lactate metabolism.
- Warn patients against excessive alcohol intake while receiving SYNJARDY.
Use in Specific Populations
Pregnancy
Risk Summary
- Based on animal data showing adverse renal effects, SYNJARDY is not recommended during the second and third trimesters of pregnancy.
- Limited available data with SYNJARDY or empagliflozin in pregnant women are not sufficient to determine a drug-associated risk for major birth defects and miscarriage.
- Published studies with metformin use during pregnancy have not reported a clear association with metformin and major birth defect or miscarriage risk.
- There are risks to the mother and fetus associated with poorly controlled diabetes in pregnancy.
- In animal studies, adverse renal changes were observed in rats when empagliflozin was administered during a period of renal development corresponding to the late second and third trimesters of human pregnancy.
- Doses approximately 13-times the maximum clinical dose caused renal pelvic and tubule dilatations that were reversible.
- Empagliflozin was not teratogenic in rats and rabbits up to 300 mg/kg/day, which approximates 48-times and 128-times, respectively, the maximum clinical dose of 25 mg when administered during organogenesis.
- No adverse developmental effects were observed when metformin was administered to pregnant Sprague Dawley rats and rabbits during the period of organogenesis at doses up to 2- and 6-times, respectively, a 2000 mg clinical dose, based on body surface area.
- The estimated background risk of major birth defects is 6-10% in women with pre-gestational diabetes with a HbA1c >7 and has been reported to be as high as 20-25% in women with HbA1c >10.
- The estimated background risk of miscarriage for the indicated population is unknown.
- In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Empagliflozin and metformin hydrochloride in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Empagliflozin and metformin hydrochloride during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Empagliflozin and metformin hydrochloride in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Empagliflozin and metformin hydrochloride in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Empagliflozin and metformin hydrochloride in geriatric settings.
Gender
There is no FDA guidance on the use of Empagliflozin and metformin hydrochloride with respect to specific gender populations.
Race
There is no FDA guidance on the use of Empagliflozin and metformin hydrochloride with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Empagliflozin and metformin hydrochloride in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Empagliflozin and metformin hydrochloride in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Empagliflozin and metformin hydrochloride in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Empagliflozin and metformin hydrochloride in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Empagliflozin and metformin hydrochloride Administration in the drug label.
Monitoring
There is limited information regarding Empagliflozin and metformin hydrochloride Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Empagliflozin and metformin hydrochloride and IV administrations.
Overdosage
There is limited information regarding Empagliflozin and metformin hydrochloride overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Empagliflozin and metformin hydrochloride Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Empagliflozin and metformin hydrochloride Mechanism of Action in the drug label.
Structure
There is limited information regarding Empagliflozin and metformin hydrochloride Structure in the drug label.
Pharmacodynamics
There is limited information regarding Empagliflozin and metformin hydrochloride Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Empagliflozin and metformin hydrochloride Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Empagliflozin and metformin hydrochloride Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Empagliflozin and metformin hydrochloride Clinical Studies in the drug label.
How Supplied
There is limited information regarding Empagliflozin and metformin hydrochloride How Supplied in the drug label.
Storage
There is limited information regarding Empagliflozin and metformin hydrochloride Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Empagliflozin and metformin hydrochloride |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Empagliflozin and metformin hydrochloride |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Empagliflozin and metformin hydrochloride Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Empagliflozin and metformin hydrochloride interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Empagliflozin and metformin hydrochloride Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Empagliflozin and metformin hydrochloride Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.