Doxepin (topical): Difference between revisions
(Created page with "{{DrugProjectFormSinglePage |authorTag= <!--Overview--> |genericName= |aOrAn= a |drugClass= |indication= |hasBlackBoxWarning= Yes |adverseReactions= <!--Bla...") |
No edit summary |
||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag= | |authorTag= | ||
<!--Overview--> | <!--Overview--> | ||
|genericName= | |genericName= | ||
|aOrAn= | |aOrAn= | ||
a | a | ||
|drugClass= | |drugClass= | ||
|indication= | |indication= | ||
|hasBlackBoxWarning= | |hasBlackBoxWarning= | ||
|adverseReactions= | |adverseReactions= | ||
<!--Black Box Warning--> | <!--Black Box Warning--> | ||
|blackBoxWarningTitle= | |blackBoxWarningTitle= | ||
Title | Title | ||
|blackBoxWarningBody= | |blackBoxWarningBody= | ||
<i><span style="color:#FF0000;">ConditionName: </span></i> | <i><span style="color:#FF0000;">ConditionName: </span></i> | ||
| Line 42: | Line 24: | ||
<!--FDA-Labeled Indications and Dosage (Adult)--> | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
|fdaLIADAdult= | |fdaLIADAdult= | ||
*Doxepin Cream is indicated for the short-term (up to 8 days) management of moderate pruritus in adult patients with atopic dermatitis or lichen simplex chronicus | |||
====Dosage==== | |||
*A thin film of doxepin Cream should be applied four times each day with at least a 3 to 4 hour interval between applications. There are no data to establish the safety and effectiveness of doxepin Cream when used for greater than 8 days. Chronic use beyond eight days may result in higher systemic levels and should be avoided. Use of doxepin Cream for longer than 8 days may result in an increased likelihood of contact sensitization. | |||
*The risk for sedation may increase with greater body surface area application of doxepin Cream . Clinical experience has shown that drowsiness is significantly more common in patients applying doxepin Cream to over 10% of body surface area; therefore, patients with greater than 10% of body surface area affected should be particularly cautioned concerning possible drowsiness and other systemic adverse effects of doxepin. If excessive drowsiness occurs, it may be necessary to do one or more of the following: reduce the body surface area treated, reduce the number of applications per day, reduce the amount of cream applied, or discontinue the drug. | |||
*Occlusive dressings may increase the absorption of most topical drugs; therefore, occlusive dressings should not be utilized with doxepin Cream. | |||
<!--Off-Label Use and Dosage (Adult)--> | <!--Off-Label Use and Dosage (Adult)--> | ||
<!--Guideline-Supported Use (Adult)--> | <!--Guideline-Supported Use (Adult)--> | ||
|offLabelAdultGuideSupport= | |offLabelAdultGuideSupport= | ||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
<!--Non–Guideline-Supported Use (Adult)--> | <!--Non–Guideline-Supported Use (Adult)--> | ||
|offLabelAdultNoGuideSupport= | |offLabelAdultNoGuideSupport= | ||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
| Line 68: | Line 48: | ||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | <!--FDA-Labeled Indications and Dosage (Pediatric)--> | ||
|fdaLIADPed= | |fdaLIADPed= | ||
There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | ||
| Line 78: | Line 55: | ||
<!--Guideline-Supported Use (Pediatric)--> | <!--Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedGuideSupport= | |offLabelPedGuideSupport= | ||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
<!--Non–Guideline-Supported Use (Pediatric)--> | <!--Non–Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedNoGuideSupport= | |offLabelPedNoGuideSupport= | ||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
<!--Contraindications--> | <!--Contraindications--> | ||
|contraindications= | |contraindications= | ||
* | *Because doxepin HCl has an anticholinergic effect and because significant plasma levels of doxepin are detectable after topical doxepin Cream application, the use of doxepin Cream is contraindicated in patients with untreated narrow angle glaucoma or a tendency to urinary retention. | ||
*doxepin Cream is contraindicated in individuals who have shown previous sensitivity to any of its components. | |||
<!--Warnings--> | <!--Warnings--> | ||
|warnings= | |||
*Drowsiness occurs in over 20% of patients treated with doxepin Cream, especially in patients receiving treatment to greater than 10% of their body surface area. Patients should be warned about the possibility of sedation and cautioned against driving a motor vehicle or operating hazardous machinery while being treated with doxepin Cream. | |||
*The sedating effects of alcoholic beverages, antihistamines, and other CNS depressants may be potentiated when doxepin Cream is used. | |||
*If excessive drowsiness occurs it may be necessary to reduce the frequency of applications, the amount of cream applied, and/or the percentage of body surface area treated, or discontinue the drug. However, the efficacy with reduced frequency of applications has not been established. | |||
* | *Keep this product away from the eyes. | ||
====Precautions==== | ====Precautions==== | ||
* | *General Drowsiness: Since drowsiness may occur with the use of doxepin Cream, patients should be warned of the possibility and cautioned against driving a car or operating dangerous machinery while using this drug. Patients should also be cautioned that their response to alcohol may be potentiated. | ||
*Sedating drugs may cause confusion and oversedation in the elderly; elderly patients generally should be observed closely for confusion and oversedation when started on doxepin Cream. | |||
Use under occlusion: Occlusive dressings may increase the absorption of most topical drugs; therefore, occlusive dressings should not be utilized with doxepin Cream. | |||
*Contact sensitization: Use of doxepin Cream can cause Type IV hypersensitivity reactions (contact sensitization) to doxepin. | |||
<!--Adverse Reactions--> | <!--Adverse Reactions--> | ||
<!--Clinical Trials Experience--> | <!--Clinical Trials Experience--> | ||
|clinicalTrials= | |||
*Controlled Clinical Trials | |||
:*Systemic Adverse Effects: In controlled clinical trials of patients treated with doxepin Cream, the most common systemic adverse event reported was drowsiness. Drowsiness occurred in 71 of 330 (22%) of patients treated with doxepin Cream compared to 7 of 334 (2%) of patients treated with vehicle cream. Drowsiness resulted in the premature discontinuation of the drug in approximately 5% of patients treated with doxepin Cream in controlled clinical trials. | |||
:*Local Site Adverse Effects: In controlled clinical trials of patients treated with doxepin Cream, the most common local site adverse event reported was burning and/or stinging at the site of application. These occurred in 76 of 330 (23%) of patients treated with doxepin Cream compared to 54 of 334 (16%) of patients treated with vehicle cream. Most of these reactions were categorized as "mild"; however, approximately 25% of patients who reported burning and/or stinging reported the reaction as "severe". Four patients treated with doxepin Cream withdrew from the study because of the burning and/or stinging. | |||
:*The table below presents the adverse events reported at an incidence of ≥ 1 % in either doxepin or vehicle cream treatment groups during the trials: | |||
: [[File:{{PAGENAME}}02.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
:*Adverse events occurring in 0.5% to < 1.0% of doxepin Cream treated patients in the controlled clinical trials included: nervousness/anxiety, tongue numbness, fever, and nausea. | |||
<!--Postmarketing Experience--> | <!--Postmarketing Experience--> | ||
|postmarketing= | |||
*Twenty-six cases of allergic contact dermatitis have been reported in patients using doxepin Cream, twenty of which were documented by positive patch test to doxepin 5% cream. | |||
<!--Drug Interactions--> | |||
|drugInteractions= | |||
* Studies have not been performed examining drug interactions with doxepin Cream. However, since plasma levels of doxepin following topical application of doxepin Cream can reach levels obtained with oral doxepin HCl therapy, the following drug interactions are possible following topical doxepin Cream application: | |||
Drugs Metabolized by P450 2D6: The biochemical activity of the drug metabolizing isozyme cytochrome P450 2D6 (debrisoquin hydroxylase) is reduced in a subset of the Caucasian population (about 7-10% of Caucasians are so-called "poor metabolizers"); reliable estimates of the prevalence of reduced P450 2D6 isozyme activity among Asian, African and other populations are not yet available. Poor metabolizers have higher than expected plasma concentrations of tricyclic antidepressants (TCAs) when given usual doses. Depending on the fraction of drug metabolized by P450 2D6, the increase in plasma concentration may be small, or quite large (8-fold increase in plasma AUC of the TCA). | |||
*In addition, certain drugs inhibit the activity of this isozyme and make normal metabolizers resemble poor metabolizers. An individual who is stable on a given dosage regimen of a TCA may become abruptly toxic when given one of these inhibiting drugs as concomitant therapy. The drugs that inhibit cytochrome P450 2D6 include some that are not metabolized by the enzyme (quinidine; cimetidine) and many that are substrates for P450 2D6 (many other antidepressants, phenothiazines, and the Type 1C antiarrhythmics propafenone and flecainide). While all the selective serotonin reuptake inhibitors (SSRIs), e.g., fluoxetine, sertraline, and paroxetine, inhibit P450 2D6, they may vary in the extent of inhibition. The extent to which SSRI-TCA interactions may pose clinical problems will depend on the degree of inhibition and the pharmacokinetics of the SSRI involved. Nevertheless, caution is indicated in the co-administration of TCAs with any of the SSRIs. Of particular importance, sufficient time must elapse before initiating TCA treatment in a patient being withdrawn from fluoxetine, given the long half-life of the parent and active metabolite (at least 5 weeks may be necessary). | |||
*Concomitant use of tricyclic antidepressants with drugs that can inhibit cytochrome P450 2D6 may require lower doses than usually prescribed for either the tricyclic antidepressant or the other drug. It is desirable to monitor TCA plasma levels whenever a TCA is going to be co-administered with another drug known to be an inhibitor of P450 2D6. | |||
*MAO Inhibitors: Serious side effects and even death have been reported following the concomitant use of certain drugs with MAO inhibitors. Therefore, MAO inhibitors should be discontinued at least two weeks prior to the cautious initiation of therapy with doxepin Cream. The exact length of time may vary and is dependent upon the particular MAO inhibitor being used, the length of time it has been administered, and the dosage involved. | |||
*Cimetidine: Serious anticholinergic symptoms (i.e., severe dry mouth, urinary retention and blurred vision) have been associated with elevations in the serum levels of tricyclic antidepressant when cimetidine therapy is initiated. Additionally, higher than expected tricyclic antidepressant levels have been observed when they are begun in patients already taking cimetidine. | |||
*Alcohol: Alcohol ingestion may exacerbate the potential sedative effects of doxepin Cream. This is especially important in patients who may use alcohol excessively. | |||
* | *Tolazamide: A case of severe hypoglycemia has been reported in a type II diabetic patient maintained on tolazamide (1 gm/day) 11 days after the addition of oral doxepin (75 mg/day). | ||
: | |||
<!--Use in Specific Populations--> | <!--Use in Specific Populations--> | ||
|useInPregnancyFDA= | |useInPregnancyFDA= | ||
|useInPregnancyAUS= | |useInPregnancyAUS= | ||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | ||
|useInLaborDelivery= | |useInLaborDelivery= | ||
There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | ||
|useInNursing= | |||
*Doxepin is excreted in human milk after oral administration. It is possible that doxepin may also be excreted in human milk following topical application of doxepin Cream. | |||
*One case has been reported of apnea and drowsiness in a nursing infant whose mother was taking an oral dosage form of doxepin HCl. | |||
*Because of the potential for serious adverse reactions in nursing infants from doxepin, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. | |||
|useInPed= | |useInPed= | ||
*The use of doxepin Cream in pediatric patients is not recommended. Safe conditions for use of doxepin Cream in children have not been established. One case has been reported of a 2.5 year old child who developed somnolence, grand mal seizure, respiratory depression, ECG abnormalities, and coma after treatment with doxepin Cream. A total of 27 grams had been applied over three days for eczema. He was treated with supportive care, activated charcoal, and systemic alkalization and recovered. | |||
|useInGeri= | |||
*Clinical studies of doxepin Cream did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy. | |||
*The extent of renal excretion of doxepin has not been determined. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selections. | |||
*Sedating drugs may cause confusion and oversedation in the elderly; elderly patients generally should be observed closely for confusion and oversedation when started on doxepin Cream. An 80-year old male nursing home patient developed probable systemic anticholinergic toxicity which included urinary retention and delirium after doxepin Cream had been applied to his arms, legs and back three times daily for two days. | |||
|useInGender= | |useInGender= | ||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | ||
|useInRace= | |useInRace= | ||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | ||
|useInRenalImpair= | |useInRenalImpair= | ||
There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | ||
|useInHepaticImpair= | |useInHepaticImpair= | ||
There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | ||
|useInReproPotential= | |useInReproPotential= | ||
There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | ||
|useInImmunocomp= | |useInImmunocomp= | ||
There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | ||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration= | |administration= | ||
* | * Topical | ||
|monitoring= | |monitoring= | ||
| Line 183: | Line 172: | ||
There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | ||
<!--IV Compatibility--> | <!--IV Compatibility--> | ||
|IVCompat= | |IVCompat= | ||
| Line 192: | Line 180: | ||
<!--Overdosage--> | <!--Overdosage--> | ||
|overdose= | |||
*Deaths may occur from overdosage with this class of drugs. As the management is complex and changing, it is recommended that the physician contact a poison control center for current information on treatment. Signs and symptoms of toxicity develop rapidly after tricyclic antidepressant overdose; therefore, hospital monitoring is required as soon as possible. | |||
*Manifestations | |||
:*Should overdosage with topical application of doxepin Cream occur, the signs and symptoms may include: cardiac dysrhythmias, severe hypotension, convulsions, and CNS depression, including coma. Changes in the electrocardiogram, particularly in QRS axis or width, are clinically significant indicators of tricyclic antidepressant toxicity. | |||
:*Other signs of overdose may include: confusion, disturbed concentration, transient visual hallucinations, dilated pupils, agitation, hyperactive reflexes, stupor, drowsiness, muscle rigidity, vomiting, hypothermia, hyperpyrexia, or any of the symptoms listed under ADVERSE REACTIONS. | |||
*General Recommendations | |||
:*General: Obtain an ECG and immediately initiate cardiac monitoring. Protect the patient's airway, establish an intravenous line and initiate gastric decontamination. A minimum of six hours of observation with cardiac monitoring and observation for signs of CNS or respiratory depression, hypotension, cardiac dysrhythmias and/or conduction blocks, and seizures is strongly advised. If signs of toxicity occur at any time during this period, extended monitoring is recommended. There are case reports of patients succumbing to fatal dysrhythmias late after overdose; these patients had clinical evidence of significant poisoning prior to death and most received inadequate gastrointestinal decontamination. Monitoring of plasma drug levels should not guide management of the patient. | |||
:*Cardiovascular: A maximal limb-lead QRS duration of >/=0.10 seconds may be the best indication of the severity of the overdose. Intravenous sodium bicarbonate should be used to maintain the serum pH in the range of 7.45 to 7.55. If the pH response is inadequate, hyperventilation may also be used. Concomitant use of hyperventilation and sodium bicarbonate should be done with extreme caution, with frequent pH monitoring. A pH>7.60 or a pCO2 <20 mm Hg is undesirable. Dysrhythmias unresponsive to sodium bicarbonate therapy/hyperventilation may respond to lidocaine, bretylium or phenytoin. Type 1A and 1C antiarrhythmics are generally contraindicated (e.g., quinidine, disopyramide, and procainamide). | |||
:*In rare instances, hemoperfusion may be beneficial in acute refractory cardiovascular instability in patients with acute toxicity. However, hemodialysis, peritoneal dialysis, exchange transfusions, and forced diuresis generally have been reported as ineffective in tricyclic antidepressant poisoning. | |||
:*CNS: In patients with CNS depression, early intubation is advised because of the potential for abrupt deterioration. Seizures should be controlled with benzodiazepines, or if these are ineffective, other anticonvulsants (e.g., phenobarbital, phenytoin). Physostigmine is not recommended except to treat life-threatening symptoms that have been unresponsive to other therapies, and then only in consultation with a poison control center. | |||
:*Pediatric Management: The principles of management of child and adult overdosages are similar. It is strongly recommended that the physician contact the local poison control center for specific pediatric treatment. | |||
<!--Pharmacology--> | <!--Pharmacology--> | ||
<!--Drug box 2--> | <!--Drug box 2--> | ||
|drugBox={{Drugbox2 | |||
| Verifiedfields = changed | |||
| Watchedfields = changed | |||
| verifiedrevid = 459441838 | |||
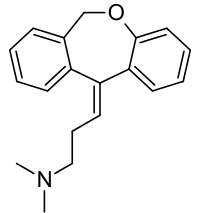
| IUPAC_name = (''E''/''Z'')-3-(dibenzo[''b,e'']oxepin-11(6''H'')-ylidene)-''N,N''-dimethylpropan-1-amine | |||
| image = Doxepin2DACS.svg.png | |||
| width = 200 | |||
| image2 = Doxepin3Dan.gif | |||
| width2 = 250 | |||
| | <!--Clinical data--> | ||
| tradename = Sinequan, Zonalon | |||
| Drugs.com = {{drugs.com|monograph|doxepin-hydrochloride}} | |||
| MedlinePlus = a682390 | |||
| licence_US = Doxepin | |||
| pregnancy_AU = C | |||
| pregnancy_US = B | |||
| legal_AU = S4 | |||
| legal_CA = Rx-only | |||
| legal_UK = POM | |||
| legal_US = Rx-only | |||
| routes_of_administration = [[oral administration|Oral]], topical, intravenous, intramuscular | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = | |||
| protein_bound = 76%<ref name = EMC/> | |||
| metabolism = [[Hepatic]] ([[CYP2D6]], [[CYP2C19]], [[CYP1A2]],& [[CYP3A4]] mediated)<ref>Product Information: ZONALON(R) cream, doxepin hcl cream. Doak Dermatologics, Fairfield, NJ, 2005.</ref><ref>Product Information: SILENOR(R) oral tablets, doxepin oral tablets. Somaxon Pharmaceuticals, Inc., San Diego, CA, 2010.</ref> | |||
| elimination_half-life = 8-24 hr (mean 17 hours); 31 hr for active metabolite, desmethyldoxepin<ref name = EMC>{{cite web|title=Sinepin Capsules 25mg - Summary of Product Characteristics (SPC)|work=electronic Medicines Compendium|publisher=Marlborough Pharmaceuticals Ltd|date=22 September 2011|accessdate=3 December 2013|url=http://www.medicines.org.uk/emc/medicine/24084/SPC/Sinepin+Capsules+25mg/}}</ref> | |||
| excretion = Urine | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 1668-19-5 | |||
| ATC_prefix = N06 | |||
| ATC_suffix = AA12 | |||
| PubChem = 3158 | |||
| IUPHAR_ligand = 1225 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB01142 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 3046 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = 5ASJ6HUZ7D | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D07875 | |||
| ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ChEBI = 4710 | |||
| ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| ChEMBL = 101740 | |||
<!--Chemical data--> | |||
| C=19 | H=21 | N=1 | O=1 | |||
| molecular_weight = 279.376 g/mol | |||
| smiles = CN(C)CC/C=C1C2=C(C=CC=C2)OCC3=C/1C=CC=C3 | |||
| InChI = 1/C19H21NO/c1-20(2)13-7-11-17-16-9-4-3-8-15(16)14-21-19-12-6-5-10-18(17)19/h3-6,8-12H,7,13-14H2,1-2H3 | |||
| InChIKey = ODQWQRRAPPTVAG-UHFFFAOYAU | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C19H21NO/c1-20(2)13-7-11-17-16-9-4-3-8-15(16)14-21-19-12-6-5-10-18(17)19/h3-6,8-12H,7,13-14H2,1-2H3 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = ODQWQRRAPPTVAG-UHFFFAOYSA-N | |||
}} | |||
<!--Mechanism of Action--> | <!--Mechanism of Action--> | ||
|mechAction= | |mechAction= | ||
<!--Structure--> | <!--Structure--> | ||
|structure= | |structure= | ||
: [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | : [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
<!--Pharmacodynamics--> | <!--Pharmacodynamics--> | ||
|PD= | |PD= | ||
| Line 228: | Line 280: | ||
<!--Pharmacokinetics--> | <!--Pharmacokinetics--> | ||
|PK= | |PK= | ||
| Line 234: | Line 285: | ||
<!--Nonclinical Toxicology--> | <!--Nonclinical Toxicology--> | ||
|nonClinToxic= | |||
*Carcinogenesis, Mutagenesis, Impairment of Fertility | |||
:*Carcinogenesis, mutagenesis, and impairment of fertility studies have not been conducted with doxepin hydrochloride. | |||
:*Pregnancy Category B: Reproduction studies have been performed in which doxepin was orally administered to rats and rabbits at doses up to 0.6 and 1.2 times, respectively, the estimated exposure to doxepin that results from use of 16 grams of doxepin Cream per day (four applications of four grams of cream per day; dose multiples reflect comparisons made following normalization of the data on the basis of body surface area estimates) and have revealed no evidence of harm to rat or rabbit fetuses due to doxepin. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. | |||
<!--Clinical Studies--> | <!--Clinical Studies--> | ||
|clinicalStudies= | |clinicalStudies= | ||
| Line 246: | Line 298: | ||
<!--How Supplied--> | <!--How Supplied--> | ||
|howSupplied= | |howSupplied= | ||
* | * doxepin Cream is available in a 45 g (NDC 0064-3600-45) tube. Store at or below 27° C (80° F). | ||
<!--Patient Counseling Information--> | <!--Patient Counseling Information--> | ||
|fdaPatientInfo= | |fdaPatientInfo= | ||
| Line 258: | Line 308: | ||
<!--Precautions with Alcohol--> | <!--Precautions with Alcohol--> | ||
|alcohol= | |alcohol= | ||
| Line 264: | Line 313: | ||
<!--Brand Names--> | <!--Brand Names--> | ||
|brandNames= | |brandNames= | ||
<!--Look-Alike Drug Names--> | <!--Look-Alike Drug Names--> | ||
|lookAlike= | |lookAlike= | ||
<!--Drug Shortage Status--> | <!--Drug Shortage Status--> | ||
|drugShortage= | |drugShortage= | ||
}} | }} | ||
<!--Pill Image--> | <!--Pill Image--> | ||
<!--Label Display Image--> | <!--Label Display Image--> | ||
{{LabelImage | {{LabelImage | ||
|fileName={{PAGENAME}} | |fileName={{PAGENAME}}03.png|This image is provided by the National Library of Medicine. | ||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
|fileName={{PAGENAME}} | |fileName={{PAGENAME}}04.png|This image is provided by the National Library of Medicine. | ||
}} | }} | ||
Revision as of 14:15, 12 May 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Doxepin (topical) is a that is FDA approved for the {{{indicationType}}} of . Common adverse reactions include .
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Doxepin Cream is indicated for the short-term (up to 8 days) management of moderate pruritus in adult patients with atopic dermatitis or lichen simplex chronicus
Dosage
- A thin film of doxepin Cream should be applied four times each day with at least a 3 to 4 hour interval between applications. There are no data to establish the safety and effectiveness of doxepin Cream when used for greater than 8 days. Chronic use beyond eight days may result in higher systemic levels and should be avoided. Use of doxepin Cream for longer than 8 days may result in an increased likelihood of contact sensitization.
- The risk for sedation may increase with greater body surface area application of doxepin Cream . Clinical experience has shown that drowsiness is significantly more common in patients applying doxepin Cream to over 10% of body surface area; therefore, patients with greater than 10% of body surface area affected should be particularly cautioned concerning possible drowsiness and other systemic adverse effects of doxepin. If excessive drowsiness occurs, it may be necessary to do one or more of the following: reduce the body surface area treated, reduce the number of applications per day, reduce the amount of cream applied, or discontinue the drug.
- Occlusive dressings may increase the absorption of most topical drugs; therefore, occlusive dressings should not be utilized with doxepin Cream.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Doxepin (topical) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Doxepin (topical) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Doxepin (topical) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Doxepin (topical) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Doxepin (topical) in pediatric patients.
Contraindications
- Because doxepin HCl has an anticholinergic effect and because significant plasma levels of doxepin are detectable after topical doxepin Cream application, the use of doxepin Cream is contraindicated in patients with untreated narrow angle glaucoma or a tendency to urinary retention.
- doxepin Cream is contraindicated in individuals who have shown previous sensitivity to any of its components.
Warnings
- Drowsiness occurs in over 20% of patients treated with doxepin Cream, especially in patients receiving treatment to greater than 10% of their body surface area. Patients should be warned about the possibility of sedation and cautioned against driving a motor vehicle or operating hazardous machinery while being treated with doxepin Cream.
- The sedating effects of alcoholic beverages, antihistamines, and other CNS depressants may be potentiated when doxepin Cream is used.
- If excessive drowsiness occurs it may be necessary to reduce the frequency of applications, the amount of cream applied, and/or the percentage of body surface area treated, or discontinue the drug. However, the efficacy with reduced frequency of applications has not been established.
- Keep this product away from the eyes.
Precautions
- General Drowsiness: Since drowsiness may occur with the use of doxepin Cream, patients should be warned of the possibility and cautioned against driving a car or operating dangerous machinery while using this drug. Patients should also be cautioned that their response to alcohol may be potentiated.
- Sedating drugs may cause confusion and oversedation in the elderly; elderly patients generally should be observed closely for confusion and oversedation when started on doxepin Cream.
Use under occlusion: Occlusive dressings may increase the absorption of most topical drugs; therefore, occlusive dressings should not be utilized with doxepin Cream.
- Contact sensitization: Use of doxepin Cream can cause Type IV hypersensitivity reactions (contact sensitization) to doxepin.
Adverse Reactions
Clinical Trials Experience
- Controlled Clinical Trials
- Systemic Adverse Effects: In controlled clinical trials of patients treated with doxepin Cream, the most common systemic adverse event reported was drowsiness. Drowsiness occurred in 71 of 330 (22%) of patients treated with doxepin Cream compared to 7 of 334 (2%) of patients treated with vehicle cream. Drowsiness resulted in the premature discontinuation of the drug in approximately 5% of patients treated with doxepin Cream in controlled clinical trials.
- Local Site Adverse Effects: In controlled clinical trials of patients treated with doxepin Cream, the most common local site adverse event reported was burning and/or stinging at the site of application. These occurred in 76 of 330 (23%) of patients treated with doxepin Cream compared to 54 of 334 (16%) of patients treated with vehicle cream. Most of these reactions were categorized as "mild"; however, approximately 25% of patients who reported burning and/or stinging reported the reaction as "severe". Four patients treated with doxepin Cream withdrew from the study because of the burning and/or stinging.
- The table below presents the adverse events reported at an incidence of ≥ 1 % in either doxepin or vehicle cream treatment groups during the trials:
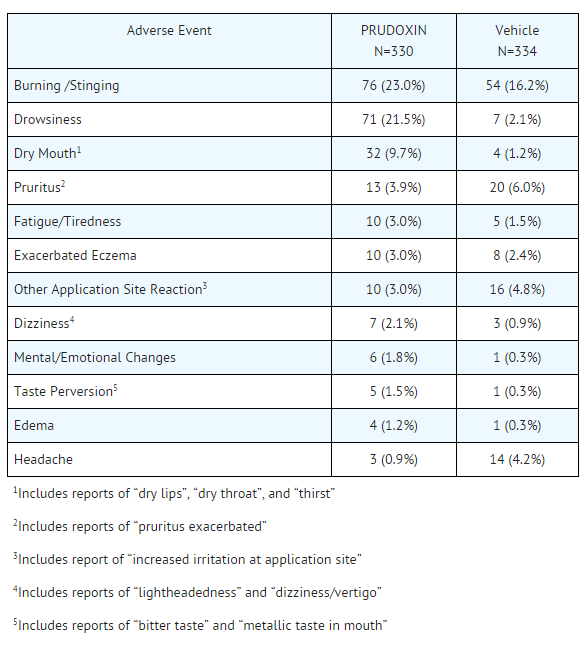
This image is provided by the National Library of Medicine.
- Adverse events occurring in 0.5% to < 1.0% of doxepin Cream treated patients in the controlled clinical trials included: nervousness/anxiety, tongue numbness, fever, and nausea.
Postmarketing Experience
- Twenty-six cases of allergic contact dermatitis have been reported in patients using doxepin Cream, twenty of which were documented by positive patch test to doxepin 5% cream.
Drug Interactions
- Studies have not been performed examining drug interactions with doxepin Cream. However, since plasma levels of doxepin following topical application of doxepin Cream can reach levels obtained with oral doxepin HCl therapy, the following drug interactions are possible following topical doxepin Cream application:
Drugs Metabolized by P450 2D6: The biochemical activity of the drug metabolizing isozyme cytochrome P450 2D6 (debrisoquin hydroxylase) is reduced in a subset of the Caucasian population (about 7-10% of Caucasians are so-called "poor metabolizers"); reliable estimates of the prevalence of reduced P450 2D6 isozyme activity among Asian, African and other populations are not yet available. Poor metabolizers have higher than expected plasma concentrations of tricyclic antidepressants (TCAs) when given usual doses. Depending on the fraction of drug metabolized by P450 2D6, the increase in plasma concentration may be small, or quite large (8-fold increase in plasma AUC of the TCA).
- In addition, certain drugs inhibit the activity of this isozyme and make normal metabolizers resemble poor metabolizers. An individual who is stable on a given dosage regimen of a TCA may become abruptly toxic when given one of these inhibiting drugs as concomitant therapy. The drugs that inhibit cytochrome P450 2D6 include some that are not metabolized by the enzyme (quinidine; cimetidine) and many that are substrates for P450 2D6 (many other antidepressants, phenothiazines, and the Type 1C antiarrhythmics propafenone and flecainide). While all the selective serotonin reuptake inhibitors (SSRIs), e.g., fluoxetine, sertraline, and paroxetine, inhibit P450 2D6, they may vary in the extent of inhibition. The extent to which SSRI-TCA interactions may pose clinical problems will depend on the degree of inhibition and the pharmacokinetics of the SSRI involved. Nevertheless, caution is indicated in the co-administration of TCAs with any of the SSRIs. Of particular importance, sufficient time must elapse before initiating TCA treatment in a patient being withdrawn from fluoxetine, given the long half-life of the parent and active metabolite (at least 5 weeks may be necessary).
- Concomitant use of tricyclic antidepressants with drugs that can inhibit cytochrome P450 2D6 may require lower doses than usually prescribed for either the tricyclic antidepressant or the other drug. It is desirable to monitor TCA plasma levels whenever a TCA is going to be co-administered with another drug known to be an inhibitor of P450 2D6.
- MAO Inhibitors: Serious side effects and even death have been reported following the concomitant use of certain drugs with MAO inhibitors. Therefore, MAO inhibitors should be discontinued at least two weeks prior to the cautious initiation of therapy with doxepin Cream. The exact length of time may vary and is dependent upon the particular MAO inhibitor being used, the length of time it has been administered, and the dosage involved.
- Cimetidine: Serious anticholinergic symptoms (i.e., severe dry mouth, urinary retention and blurred vision) have been associated with elevations in the serum levels of tricyclic antidepressant when cimetidine therapy is initiated. Additionally, higher than expected tricyclic antidepressant levels have been observed when they are begun in patients already taking cimetidine.
- Alcohol: Alcohol ingestion may exacerbate the potential sedative effects of doxepin Cream. This is especially important in patients who may use alcohol excessively.
- Tolazamide: A case of severe hypoglycemia has been reported in a type II diabetic patient maintained on tolazamide (1 gm/day) 11 days after the addition of oral doxepin (75 mg/day).
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Doxepin (topical) in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Doxepin (topical) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Doxepin (topical) during labor and delivery.
Nursing Mothers
- Doxepin is excreted in human milk after oral administration. It is possible that doxepin may also be excreted in human milk following topical application of doxepin Cream.
- One case has been reported of apnea and drowsiness in a nursing infant whose mother was taking an oral dosage form of doxepin HCl.
- Because of the potential for serious adverse reactions in nursing infants from doxepin, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- The use of doxepin Cream in pediatric patients is not recommended. Safe conditions for use of doxepin Cream in children have not been established. One case has been reported of a 2.5 year old child who developed somnolence, grand mal seizure, respiratory depression, ECG abnormalities, and coma after treatment with doxepin Cream. A total of 27 grams had been applied over three days for eczema. He was treated with supportive care, activated charcoal, and systemic alkalization and recovered.
Geriatic Use
- Clinical studies of doxepin Cream did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
- The extent of renal excretion of doxepin has not been determined. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selections.
- Sedating drugs may cause confusion and oversedation in the elderly; elderly patients generally should be observed closely for confusion and oversedation when started on doxepin Cream. An 80-year old male nursing home patient developed probable systemic anticholinergic toxicity which included urinary retention and delirium after doxepin Cream had been applied to his arms, legs and back three times daily for two days.
Gender
There is no FDA guidance on the use of Doxepin (topical) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Doxepin (topical) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Doxepin (topical) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Doxepin (topical) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Doxepin (topical) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Doxepin (topical) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Topical
Monitoring
There is limited information regarding Monitoring of Doxepin (topical) in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Doxepin (topical) in the drug label.
Overdosage
- Deaths may occur from overdosage with this class of drugs. As the management is complex and changing, it is recommended that the physician contact a poison control center for current information on treatment. Signs and symptoms of toxicity develop rapidly after tricyclic antidepressant overdose; therefore, hospital monitoring is required as soon as possible.
- Manifestations
- Should overdosage with topical application of doxepin Cream occur, the signs and symptoms may include: cardiac dysrhythmias, severe hypotension, convulsions, and CNS depression, including coma. Changes in the electrocardiogram, particularly in QRS axis or width, are clinically significant indicators of tricyclic antidepressant toxicity.
- Other signs of overdose may include: confusion, disturbed concentration, transient visual hallucinations, dilated pupils, agitation, hyperactive reflexes, stupor, drowsiness, muscle rigidity, vomiting, hypothermia, hyperpyrexia, or any of the symptoms listed under ADVERSE REACTIONS.
- General Recommendations
- General: Obtain an ECG and immediately initiate cardiac monitoring. Protect the patient's airway, establish an intravenous line and initiate gastric decontamination. A minimum of six hours of observation with cardiac monitoring and observation for signs of CNS or respiratory depression, hypotension, cardiac dysrhythmias and/or conduction blocks, and seizures is strongly advised. If signs of toxicity occur at any time during this period, extended monitoring is recommended. There are case reports of patients succumbing to fatal dysrhythmias late after overdose; these patients had clinical evidence of significant poisoning prior to death and most received inadequate gastrointestinal decontamination. Monitoring of plasma drug levels should not guide management of the patient.
- Cardiovascular: A maximal limb-lead QRS duration of >/=0.10 seconds may be the best indication of the severity of the overdose. Intravenous sodium bicarbonate should be used to maintain the serum pH in the range of 7.45 to 7.55. If the pH response is inadequate, hyperventilation may also be used. Concomitant use of hyperventilation and sodium bicarbonate should be done with extreme caution, with frequent pH monitoring. A pH>7.60 or a pCO2 <20 mm Hg is undesirable. Dysrhythmias unresponsive to sodium bicarbonate therapy/hyperventilation may respond to lidocaine, bretylium or phenytoin. Type 1A and 1C antiarrhythmics are generally contraindicated (e.g., quinidine, disopyramide, and procainamide).
- In rare instances, hemoperfusion may be beneficial in acute refractory cardiovascular instability in patients with acute toxicity. However, hemodialysis, peritoneal dialysis, exchange transfusions, and forced diuresis generally have been reported as ineffective in tricyclic antidepressant poisoning.
- CNS: In patients with CNS depression, early intubation is advised because of the potential for abrupt deterioration. Seizures should be controlled with benzodiazepines, or if these are ineffective, other anticonvulsants (e.g., phenobarbital, phenytoin). Physostigmine is not recommended except to treat life-threatening symptoms that have been unresponsive to other therapies, and then only in consultation with a poison control center.
- Pediatric Management: The principles of management of child and adult overdosages are similar. It is strongly recommended that the physician contact the local poison control center for specific pediatric treatment.
Pharmacology
Mechanism of Action
There is limited information regarding Doxepin (topical) Mechanism of Action in the drug label.
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Doxepin (topical) in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Doxepin (topical) in the drug label.
Nonclinical Toxicology
- Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenesis, mutagenesis, and impairment of fertility studies have not been conducted with doxepin hydrochloride.
- Pregnancy Category B: Reproduction studies have been performed in which doxepin was orally administered to rats and rabbits at doses up to 0.6 and 1.2 times, respectively, the estimated exposure to doxepin that results from use of 16 grams of doxepin Cream per day (four applications of four grams of cream per day; dose multiples reflect comparisons made following normalization of the data on the basis of body surface area estimates) and have revealed no evidence of harm to rat or rabbit fetuses due to doxepin. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Clinical Studies
There is limited information regarding Clinical Studies of Doxepin (topical) in the drug label.
How Supplied
- doxepin Cream is available in a 45 g (NDC 0064-3600-45) tube. Store at or below 27° C (80° F).
Storage
There is limited information regarding Doxepin (topical) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Doxepin (topical) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Doxepin (topical) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Doxepin (topical) in the drug label.
Precautions with Alcohol
- Alcohol-Doxepin (topical) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Doxepin (topical) Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Doxepin (topical) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ 1.0 1.1 "Sinepin Capsules 25mg - Summary of Product Characteristics (SPC)". electronic Medicines Compendium. Marlborough Pharmaceuticals Ltd. 22 September 2011. Retrieved 3 December 2013.
- ↑ Product Information: ZONALON(R) cream, doxepin hcl cream. Doak Dermatologics, Fairfield, NJ, 2005.
- ↑ Product Information: SILENOR(R) oral tablets, doxepin oral tablets. Somaxon Pharmaceuticals, Inc., San Diego, CA, 2010.
{{#subobject:
|Label Page=Doxepin (topical) |Label Name=Doxepin (topical)03.png
}}
{{#subobject:
|Label Page=Doxepin (topical) |Label Name=Doxepin (topical)04.png
}}