Double outlet right ventricle
| Double outlet right ventricle | |
 | |
|---|---|
| ICD-10 | Q20.1 |
| ICD-9 | 745.11 |
| OMIM | 217095 |
| DiseasesDB | 32215 |
| eMedicine | ped/2509 ped/2508 |
| MeSH | D004310 |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Associate Editor-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Associate Editor-in-Chief: Keri Shafer, M.D. [3]
Overview
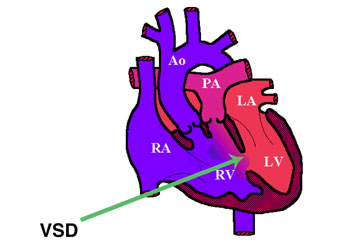
Double outlet right ventricle (DORV) is a form of congenital heart disease where both of the great arteries connect (in whole or in part) to the right ventricle (RV).
Anatomy
DORV occurs in a multiple forms, with variability of great artery position and size, as well as of ventricular septal defect (VSD) location. In general, DORV is associated with concordant atrio-ventricular connection but it can occur with or without transposition of the great arteries. The clinical manifestations are similarly variable, depending on how the anatomical defects affect the physiology of the heart, in terms of altering the normal flow of blood from the RV and left ventricle (LV) to the aorta and pulmonary artery. For example:
- in DORV with a subaortic VSD, blood from the LV flows through the VSD to the aorta and blood from the RV flows mainly to the pulmonary artery, yielding physiology similar to Tetralogy of Fallot
- in DORV with a subpulmonic VSD (called Taussig-Bing syndrome), blood from the LV flows through the VSD to the pulmonary artery and blood from the RV flows mainly to the aorta, yielding physiology similar to Transposition of the Great Arteries
- but if there is pulmonic stenosis in addition, physiology resembles Tetralogy of Fallot
- in other forms of DORV, blood from both ventricles is substantially mixed in the RV, yielding physiology that resembles a large VSD
- but again, if there is pulmonic stenosis, physiology resembles Tetralogy of Fallot
While DORV is usually associated with a ventricular septal defect (VSD), it can also be associated with an atrial septal defect (ASD), anomaly of the coronary arteries, different grades of aortic stenosis or pulmonic artery stenosis.
Pathologist define the DORV when more than 50% of both great arteries arise from the [[]right ventricle]], but surgeons use the term when 90% of the arteries arise from the right ventricle.
Epidemiology and Demographics
- The Double Outlet of the Right Ventricle (DORV) accounts for 1-1.5% of all congenital heart diseases.
- It has an incidence of 1 in 10.000 live births.
- Generally, it is diagnosed during the first month of life, and there is no sex or race predilection.
Anatomy
In the DORV, the aorta and the pulmonary artery are related to the morphologic RV. There are three essential gross morphologic features that allow the many variants to be distinguished:
The relationship of the great arteries to the ventricles
- Aorta to the right and posterior to the pulmonary trunk (normal arrangement present in a 3% of the RVOT cases).
- The great arteries side by side with the aorta to the right of the pulmonary trunk is the usual arrangement (approximately 64%)in double outlet RV. Physiologically similar to Tetralogy of Fallot.
- Aorta to the right and anterior to the pulmonary trunk (24% of the RVOT cases). Physiologically resembles TGA, specifically D-TGA with VSD.
- Aorta to the left and anterior to the pulmonary trunk (rarest arrangement representing a 7% of the RVOT cases _ L-TGA_ pattern).
The relationship of the VSD to the great arteries
- Provides the morphologic LV with its only outlet
- Subaortic
- Subpulmonic
- Beneath both (doubly committed)
- Beneath neither
The presence or absence of outflow obstruction to RV outflow
- Pulmonic stenosis occurs in 41% to 72% of patients with double outlet RV
- When pulmonic stenosis occurs, the VSD is almost always subaortic
- RV blood is diverted into the aorta with a decrease in the oxygen saturation
The most common combination is the side-by-side relationship of the great arteries, with the aorta directly to the right and with the VSD in the subaortic location.
- In the absence of pulmonic stenosis, the physiologic consequences are similar to those of an isolated VSD.
- A low pulmonary vascular resistance PVR permits RV blood to flow almost exclusively into the pulmonary trunk and permits a substantial portion of the left ventricular blood to enter the pulmonary circulation.
- Aortic O2 saturation is normal, and pulmonary artery (PA) blood flow is increased.
The Taussig-Bing anomaly involves the same great vessel relationship, but there is a subpulmonic VSD. [1] [2]
- LV blood selectively enters the pulmonary trunk because of the proximity of the subpulmonic VSD to the pulmonary artery (PA).
- Accounts for approximately 8% of cases.
Risk Factors
- Most drugs affect the development of the cardiovascular system, because it appears earlier during the embryonic period. Drugs such as busulfan, lithium, retinoids, thalidomide, and trimethadione can cause cardiac defects. Busulfan is an animal teratogen, and there are not definitive data that exposures have resulted in birth defects in humans. The other drugs are proven human teratogens, depending on the dose. The teratogenic risk of lithium is low and controversial.[3]
- Maternal use of alcohol during the first trimester of pregnancy is associated with DORV.
- Exposure to solvents during pregnancy. [4]
- A first-trimester febrile illness increases the risk by 40%–80%. [5]
- Fetal "nutrition" which is affected by maternal diet, uterine blood flow, placental function, and the fetal genome.
Pathophysiology & Etiology
The pathophysiology of the DORV is quite variable [6]. It depends on the great artery relationship (side by side, d-TGA, l-TGA, normally related arteries), the relationship of the VSD to the great arteries (subaortic or subpulmonary VSD), and on the presence or absence of RV outflow tract obstruction or pulmonary stenosis (PS).
The most common type of DORV, refers to the one with similar physiopathology than Tetralogy of Fallot and great arteries lying side by side. The aorta is located to the right of the pulmonary artery and both semilunar valves lying in the same transverse plane with the VSD in the subaortic location. If the VSD is a subpulmonic VSDis called Taussig-Bing anomaly.
The second type of DORV is the type where the aorta is anterior and to the right of the pulmonary artery (PA), funtionally resembling transposition of the great arteries (TGA), D-TGA, with a VSD. A less frequent type is where the aorta (AO) is anterior and to the left of the PA (left-transposition of the great arteries L-TGA).
In rare cases of DORV, there is a normal great artery relationship with the aorta arising posterior and to the right of the PA.
Clinical manifestations depends on the size, but mostly on the location of the VSD in relation to the great arteries. Also the clinical symptoms will depend on the presence or absence of a pulmonary stenosis.
When the VSD is subaortic, the oxygenated blood is pump from the LV into the aorta, and deoxygenated blood flow from the right atrium to the pulmonary artery (closer to its location).
Genetics
Patients with Noonan syndrome (autosomal dominant inheritance) may present with pulmonary stenosis, ASD, and restrictive cardiomyopathy. Patients with Williams syndrome have the gene manifested in a variety of body organ systems such as the cardiovascular system (associated with pulmonary stenosis, pulmonary artery stenosis, supravalvular aortic stenosis), the neurological system (mental retardation), and general manifestation such as short stature, hypercalcemia, caused by deletion at chromosome 7. [7].
Diagnosis
History and Symptoms
The patients symptoms vary greatly depending on the severity of the defect, and associated defects. The patient may be either asymptomatic or symptomatic. Newborns may not immediately exhibit problems, and some children can wait a year or so before undergoing any type of repair.
Symptoms include feeding difficulties and as a consequence poor weight gain, failure to thrive. Other sypmtoms include fatigue, difficulty breathing, and cyanosis. These symptoms are not specific for RVOT.
Physical Examination
The findings on physical examination vary significantly depending upon the presence and location of associated defects such as a VSD and pulmonic stenosis.
Subaortic VSD present, pulmonic stenosis absent
- Mild or absent cyanosis
- Volume overload to the right side is associated with CHF.
- Loud single S2
- Hyperdynamic precordium
Subaortic VSD and pulmonic stenosis both present
The major determinant of the findings is the severity of the pulmonic stenosis
- Mild Pulmonic Stenosis: mild cyanosis and mild CHF may be present, and a systolic ejection murmur of pulmonic stenosis and / or the regurgitant murmur of the VSD
- Moderate to Severe Pulmonic Stenosis: cyanosis is present
Extremities
Cyanosis and clubbing may be present
Laboratory Findings
During the initial diagnosis and management of children with double outlet right ventricle (DORV), routine laboratory studies are not required.
If polycythemia is suspected due to longstanding hypoxia, then a hemoglobin and hematocrit can be obtained.
Electrolyte and Biomarker Studies
Monitor serum electrolytes after treating children with diuretics, glycosides, and afterload-reducing agents.
Electrocardiogram
Normal sinus rhythm is generally present. There are often associated conduction defects such as a prolonged PR interval, first degree AV block. P pulmonale is present in 75% of cases. As a result of pressure and volume overload, there are signs of RV hypertrophy and there is a right axis deviation.
There are a lot of ECG variations depending on the variety of DORV the presence of associated defects. If there is a subaortic VSD with no PS a superior QRS axis (-30° to -170°) may be present with either RVH or biventricular hypertrophy and left atrial enlargement. First-degree AV block may be present with this lesion. On the other hand, if there is subpulmonic VSD or in those with subaortic VSD and PS, then right axis deviation, RVH, and often right atrial enlargement may be present. [8]
Chest X Ray
If there is mild or no pulmonary stenosis, subpulmonary or subaortic VSD, cardiomegaly may be present due to increased pulmonary blood flow. If transposition of the great arteries is present, the mediastinum may be narrow. These features are not specific for DORV.
If PS is present, chest radiography shows a normal heart size and normal-to-decreased pulmonary vascular markings.
Echocardiography
See also: Echo in Double Outlet Right Ventricle
MRI and CT
These imaging modalities allow one to determine the visceral and atrial situs as vasculo-vascular and vasculo-visceral relationships. MRI may aid in defining the spatial relationship between the VSD and the semilunar valves.
Echocardiography or Ultrasound
Editors-in-Chief: Eli V. Gelfand, MD and Keri Shafer, MD (Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA)
- Evaluation of relationship of the great arteries and right ventricle
- Possible orientations: normal, side-by-side (aorta to the right of the pulmonary artery)
- Evaluation of the ventricular septal defect
- Doppler evaluation of pressure gradients and presence of valvular regurgitation
- Associated anomalities, especially pulmonic stenosis but also:
- Atrial Septal Defect
- Subaortic Stenosis
- Patent Ductus Arteriosus
- Mitral valvular abnormalities
- Evaluation after repair
Special Echo techniques in DORV
- Optimal views:
- Parasternal long axis
- Subcostal coronal view
- Parasternal short axis
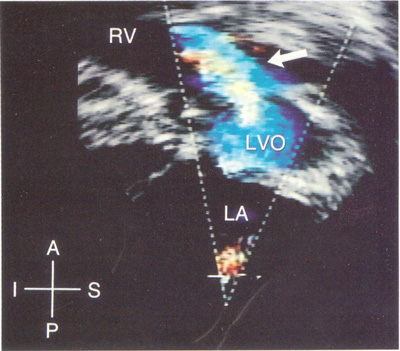
The subxiphoid view allows to characterize the origin of both great vessels emerging from the RV, and also their relationship to one another. In addition to the anatomy mentioned before it allows the evaluation of the sub-pulmonic conus, the severity of the pulmonary stenosis and the relationship of the VSD to the aortic valve.
Hemodynamic Studies
Cardiac catheterization is performed prior to complete repair, or prior to B-T shunt placement in cases of severe pulmonary stenosis.
Pathologic Findings
Images shown below are Courtesy of Professor Peter Anderson DVM PhD and Published with permission. © PEIR, University of Alabama at Birmingham, Department of Pathology
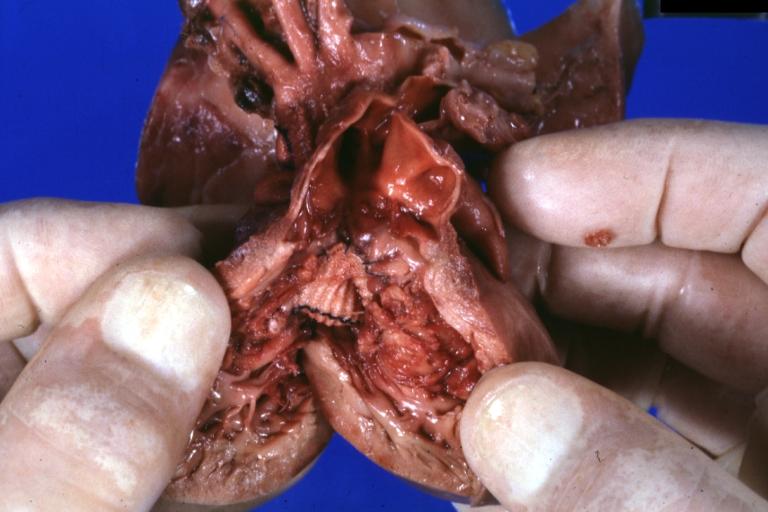
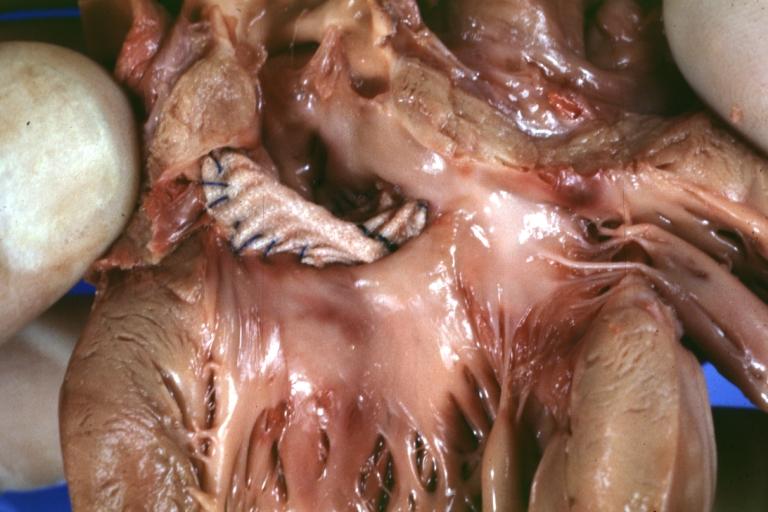
Treatment
Surgery and Device Based Therapy
If pulmonary stenosis is not present, double outlet right ventricle may be repaired in one operation. The surgeon will repair the VSD, but build a conduit through it to connect the left ventricle to the aorta. This technique is known as the Rastelli procedure. Other surgeons may opt for the arterial switch operation (or Jatene), commonly also used with transposition of the great arteries, in which the aorta is reconnected to the left ventricle
The surgical technique will depend upon the several factors: the location of the VSD and its relationship to the aortic and pulmonary valves, the presence or absence of pulmonary stenosis, and the distribution of the coronary arteries. There are also factors such as A-V discordance, size of the right ventricle, and the straddling of A-V valves.
Palliative Surgery
This approach helps to improve the patient's clinical condition, allowing him or her to gain weight to achieve optimal conditions for definitive surgical repair.
- Patients with no pulmonary stenosis + subpulmonary, subaortic, or doubly committed VSD with CHF may undergo pulmonary artery banding to decrease pulmonary blood flow.
- Patients with pulmonary stenosis + subaortic or supulmonary VSD are cyanotic with decreased pulmonary blood flow may undergo a systemic-to-pulmonary artery shunt to increase pulmonary blood flow.
Definitive Surgical Repair
The nature of the operation will depend on the relationship of VSD to the great arteries and the distribution of coronary arteries to determine surgical strategies.
If biventricular repair is not possible, a Fontan-type operation is an option with redirection of systemic (deoxygenated) blood into the pulmonary artery without compromising the ventricle. If the patients has a subpulmonary VSD, arterial switch operation with creation of an interventricular tunnel directing left ventricular outflow into the pulmonary artery, which becomes a neo-aorta due to the arterial switch. If the VSD is subaortic, a tunnel between the VSD and the aorta is made to direct oxygenated blood into systemic circulation and also to eliminate mixing of the 2 circulations. This operation is generally completed by age 4-6 months.
Most medical centers have reported an approximated in-hospital mortality after surgery of 4.8% with a late mortality of 3.2%. The more complex the defect, the higher the mortality and the need for reoperation. [9] [10] [11] [12]
Acknowledgements
The content on this page was first contributed by Dr. Leida perez
List of contributors:
References
- ↑ Double Outlet Right Ventricle, Chapter 19, in Perloff, Congenital Heart Disease, pp. 443-466.
- ↑ Silvana Horenstein, MD, Double Outlet Right Ventricle, With Transposition. e medicine.2005
- ↑ DeSantis M, Carducci B, Cavaliere AF, DeSantis L, Straface G, Caruso A. Drug-induced congenital defects. Strategies to reduce the incidence. Drug Saf.2001; 24 :889 –901.
- ↑ Suzanne M. Mone, MD et al. Effects of Environmental Exposures on the Cardiovascular System: Prenatal Period Through Adolescence. Pediatrics. vol 113. 2004.
- ↑ Tikkanen J, Heilonen OP. Maternal hyperthermia during pregnancy and cardiovascular malformations in the offspring. Eur J Epidemiol.1991; 7 :628 –635.
- ↑ Double Outlet Right Ventricle, Chapter 19, in Perloff, Congenital Heart Disease, pp. 443-466.
- ↑ CCS Consensus Conference: recommendation for the management of Adult with Congenital Heart Disease. Part 1. 2001.
- ↑ Oliver W. Caminos, M.D.Double outlet right ventricle. Chapter 21.
- ↑ Silvana Horenstein, MD, et al. Double Outlet Right Ventricle, with Transposition.e Medicine. 2006.
- ↑ Stark de Leval and M. de Leval. Surgery for Congenital Heart Defects. Grune & Stratton.
- ↑ J. Stark and M. de Laval. Surgery for Congenital Heart Defects. Grune & Stratton.
- ↑ Gatzoulis, Swan, Therrien, and Pantely. Adult Congenital Heart Disease. Blackwell Publishing.
Suggested Reading and Key General References
- Moss and Adams' Heart Disease in Infants, Children, and Adolescents Hugh D. Allen, Arthur J. Moss, David J. Driscoll, Forrest H. Adams, Timothy F. Feltes, Robert E. Shaddy, 2007 ISBN 0781786843
Suggested Links and Web Resources
- Yale Congenital Heart Disease- DORV
- Pediatric Heart Surgery
- The Congenital Heart Surgery Video Project
- Ashley: Repair of DORV
External links
de:Double outlet right Ventricle uk:Подвійне відходження магістральних судин від правого шлуночку