Dipivefrine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Dipivefrine is a Ophthalmologic Agent that is FDA approved for the treatment of of intraocular pressure in chronic open-angle glaucoma. Common adverse reactions include Burning sensation in eye, injection.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Dipivefrin hydrochloride ophthalmic solution is indicated as initial therapy for the control of intraocular pressure in chronic open-angle glaucoma. Patients responding inadequately to other antiglaucoma therapy may respond to addition of dipivefrin.
- In controlled and open-label studies of glaucoma, dipivefrin demonstrated a statistically significant intraocular pressure lowering effect. Patients using dipivefrin twice daily in studies with mean durations of 76-146 days experienced mean pressure reductions ranging from 20-24%.
- Therapeutic response to 0.1% dipivefrin twice daily is somewhat less than 2% epinephrine twice daily. Controlled studies showed statistically significant differences in lowering of intraocular pressure between 0.1% dipivefrin and 2% epinephrine. In controlled studies in patients with a history of epinephrine intolerance, only 3% of patients treated with dipivefrin exhibited intolerance, while 55% of those treated with epinephrine again developed intolerance.
- Therapeutic response to 0.1% dipivefrin twice daily is comparable to 2% pilocarpine 4 times daily. In controlled clinical studies comparing 0.1% dipivefrin and 2% pilocarpine, there were no statistically significant differences in the maintenance of IOP levels for the two medications.
- Dipivefrin does not produce miosis or accommodative spasm which cholinergic agents are known to produce. The blurred vision and night blindness often associated with miotic agents are not present with dipivefrin therapy. Patients with cataracts avoid the inability to see around lenticular opacities caused by constricted pupil.
Dosage
Initial Glaucoma Therapy
- The usual dosage of dipivefrin hydrochloride ophthalmic solution, 0.1%, is one drop in the eye(s) every 12 hours.
Replacement With Dipivefrin Hydrochloride Ophthalmic Solution
- When patients are being transferred to dipivefrin from antiglaucoma agents other than epinephrine, on the first day continue the previous medication and add one drop of dipivefrin to each eye(s) every 12 hours. On the following day, discontinue the previously used antiglaucoma agent and continue with dipivefrin.
- In transferring patients from conventional epinephrine therapy to dipivefrin, simply discontinue the epinephrine medication and institute the dipivefrin regimen.
Addition of Dipivefrin Hydrochloride Ophthalmic Solution
- When patients on other antiglaucoma agents require additional therapy, add one drop of dipivefrin every 12 hours.
Concomitant Therapy
- For difficult to control patients, the addition of dipivefrin hydrochloride ophthalmic solution to other agents such as pilocarpine, carbachol, echothiophate iodide or acetazolamide has been shown to be effective.
- Note: Not for injection.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Dipivefrine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Dipivefrine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Dipivefrine in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Dipivefrine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Dipivefrine in pediatric patients.
Contraindications
- Dipivefrin hydrochloride should not be used in patients with narrow angles since any dilation of the pupil may predispose the patient to an attack of angle-closure glaucoma. This product is contraindicated in patients who are hypersensitive to any of its components.
Warnings
- NOT FOR INJECTION – FOR OPHTHALMIC USE ONLY.
Precautions
General
- Aphakic Patients: Macular edema has been shown to occur in up to 30% of aphakic patients treated with epinephrine. Discontinuation of epinephrine generally results in reversal of the maculopathy.
Information for patients
- To avoid contamination, do not touch tip of container to the eye, eyelid, or any surface.
Animal Studies
- Rabbit studies indicated a dose-related incidence of meibomiam gland retention cysts following topical administration of both dipivefrin and epinephrine.
Adverse Reactions
Clinical Trials Experience
Cardiovascular Effects
- Tachycardia, arrhythmias and hypertension have been reported with ocular administration of epinephrine.
Local Effects
- The most frequent side effects reported with dipivefrin hydrochloride alone were injection in 6.5% of patients and burning and stinging in 6%. Follicular conjunctivitis, mydriasis and allergic reactions to dipivefrin have been reported infrequently. Epinephrine therapy can lead to adrenochrome deposits in the conjunctiva and cornea.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Dipivefrine in the drug label.
Drug Interactions
There is limited information regarding Dipivefrine Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Reproduction studies have been performed in rats and rabbits at daily oral doses up to 10 mg/kg body weight (5 mg/kg in teratogenicity studies), and have revealed no evidence of impaired fertility or harm to the fetus due to dipivefrin. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Dipivefrine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Dipivefrine during labor and delivery.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when dipivefrin hydrochloride is administered to a nursing woman.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
There is no FDA guidance on the use of Dipivefrine with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Dipivefrine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Dipivefrine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Dipivefrine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Dipivefrine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Dipivefrine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Dipivefrine in patients who are immunocompromised.
Administration and Monitoring
Administration
- Topical application
Monitoring
There is limited information regarding Monitoring of Dipivefrine in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Dipivefrine in the drug label.
Overdosage
There is limited information regarding Overdose of Dipivefrine in the drug label.
Pharmacology
| |
Dipivefrine
| |
| Systematic (IUPAC) name | |
| [2-(2,2-dimethylpropanoyloxy)-4- (1-hydroxy-2-methylamino-ethyl)- phenyl] 2,2-dimethylpropanoate | |
| Identifiers | |
| CAS number | |
| ATC code | S01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 351.437 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
B(US) |
| Legal status | |
| Routes | ? |
Mechanism of Action
- Dipivefrin is converted to epinephrine inside the human eye by enzyme hydrolysis. The liberated epinephrine, an adrenergic agonist, appears to exert its action by decreasing aqueous production and by enhancing outflow facility.
Structure
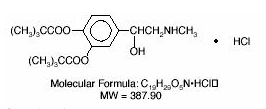
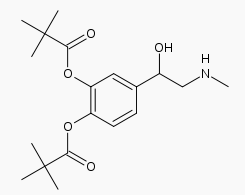
- Dipivefrin Hydrochloride Ophthalmic Solution USP, 0.1% is a sterile, isotonic solution. Dipivefrin hydrochloride is a white, crystalline powder, freely soluble in water. It is classified as a sympathomimetic agent and has the following structural formula:
- Chemical Name: (±) -3, 4-Dihydroxy-a- [(methylamino) methyl] benzyl alcohol 3, 4-dipivalate hydrochloride.
- Contains: Active: Dipivefrin hydrochloride, 0.1% (1 mg/mL). Preservative: Benzalkonium chloride 0.005%. Inactive Ingredients: Edetate Disodium, Sodium Chloride, Hydrochloric Acid and/or Sodium Hydroxide (to adjust pH to 2.5 to 3.5), Purified Water. DM-00
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Dipivefrine in the drug label.
Pharmacokinetics
- Dipivefrin hydrochloride is a member of a class of drugs known as prodrugs. Prodrugs are usually not active in themselves and require biotransformation to the parent compound before therapeutic activity is seen. These modifications are undertaken to enhance absorption, decrease side effects and enhance stability and comfort, thus making the parent compound a more useful drug. Enhanced absorption makes the prodrug a more efficient delivery system for the parent drug because less drug will be needed to produce the desired therapeutic response.
- Dipivefrin is a prodrug of epinephrine formed by the diesterification of epinephrine and pivalic acid. The addition of pivaloyl groups to the epinephrine molecule enhances its lipophilic character and, as a consequence, its penetration into the anterior chamber.
- Dipivefrin is converted to epinephrine inside the human eye by enzyme hydrolysis. The liberated epinephrine, an adrenergic agonist, appears to exert its action by decreasing aqueous production and by enhancing outflow facility. The dipivefrin hydrochloride prodrug delivery system is a more efficient way of delivering the therapeutic effects of epinephrine, with fewer side effects than are associated with conventional epinephrine therapy.
- The onset of action with one drop of dipivefrin hydrochloride ophthalmic solution occurs about 30 minutes after treatment, with maximum effect seen at about one hour.
- Using a prodrug means that less drug is needed for therapeutic effect since absorption is enhanced with the prodrug. Dipivefrin hydrochloride, 0.1% was judged less irritating than a 1% solution of epinephrine hydrochloride or bitartrate. In addition, only 8 of 455 patients (1.8%) treated with dipivefrin reported discomfort due to photophobia, glare or light sensitivity.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Dipivefrine in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Dipivefrine in the drug label.
How Supplied
- Dipivefrin Hydrochloride Ophthalmic Solution USP, 0.1% is supplied sterile in opaque plastic DROP-TAINER® Dispensers as follows:
- 5 mL–NDC 61314-235-05
- 10 mL–NDC 61314-235-10
- 15 mL–NDC-61314-235-15
Storage
- Store at controlled room temperature 15°-30°C (59°-86°F) in a tight, light-resistant container.
Images
Drug Images
{{#ask: Page Name::Dipivefrine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
Ingredients and Appearance
{{#ask: Label Page::Dipivefrine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- To avoid contamination, do not touch tip of container to the eye, eyelid, or any surface.
Precautions with Alcohol
- Alcohol-Dipivefrine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Propine®[1]
Look-Alike Drug Names
There is limited information regarding Dipivefrine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.