Diphenhydramine: Difference between revisions
Adeel Jamil (talk | contribs) No edit summary |
Adeel Jamil (talk | contribs) No edit summary |
||
| Line 6: | Line 6: | ||
|drugClass=Analgesic and antihistamine | |drugClass=Analgesic and antihistamine | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication=temporarily relieves symptoms of the common cold like | |indication=temporarily relieves symptoms of the [[common cold]] like | ||
[[Runny nose]], [[Sneezing]], [[Itchy]] watery [[eyes]], [[Itchy]] nose or [[throat]] | |||
|adverseReactions=[[Xerostomia]], [[dizziness]], [[dyskinesia]], [[sedation]], [[somnolence]], dryness of nasal mucosa, [[pharyngeal dryness]], thick sputum. | |adverseReactions=[[Xerostomia]], [[dizziness]], [[dyskinesia]], [[sedation]], [[somnolence]], dryness of [[nasal mucosa]], [[pharyngeal dryness]], thick sputum. | ||
|blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | |blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | |blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | ||
| Line 18: | Line 18: | ||
<!--FDA-Labeled Indications and Dosage (Adult)--> | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
|fdaLIADAdult=* Temporarily relieves these symptoms due to hay fever or other upper respiratory allergies: | |fdaLIADAdult=* Temporarily relieves these symptoms due to hay fever or other upper respiratory allergies: | ||
:* Runny nose | :* [[Runny nose]] | ||
:* Sneezing | :* [[Sneezing]] | ||
:* Itchy, watery eyes | :* [[Itchy]], watery [[eyes]] | ||
:* Itchy nose or throat | :* [[Itchy]] nose or [[throat]] | ||
* Temporarily relieves these symptoms of the common cold: | * Temporarily relieves these symptoms of the [[common cold]]: | ||
:* Runny nose | :* [[Runny nose]] | ||
:* Sneezing | :* [[Sneezing]] | ||
* Dosing Information | * Dosing Information | ||
| Line 32: | Line 32: | ||
[[File:Diphenhydramine dosage table01.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | [[File:Diphenhydramine dosage table01.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
|offLabelAdultGuideSupport=* Chemotherapy-induced nausea and vomiting. | |offLabelAdultGuideSupport=* [[Chemotherapy]]-induced [[nausea]] and [[vomiting]]. | ||
* Extrapyramidal disease - Medication-induced movement disorder. | * [[Extrapyramidal]] [[disease]] - [[Medication]]-induced [[movement disorder]]. | ||
* Hyperemesis gravidarum. | * [[Hyperemesis gravidarum]]. | ||
* Local anesthesia | * [[Local anesthesia]] | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Diphenhydramine in adult patients. | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Diphenhydramine in adult patients. | ||
| Line 60: | Line 60: | ||
<!--Contraindications--> | <!--Contraindications--> | ||
|contraindications=* Diphenhydramine is contraindicated in persons with known hypersensitivity. | |contraindications=* Diphenhydramine is [[contraindicated]] in persons with known [[hypersensitivity]]. | ||
|warnings=* Do not use with any other product containing diphenhydramine, even one used on skin | |warnings=* Do not use with any other product containing diphenhydramine, even one used on [[skin]] | ||
* To make a child sleepy | * To make a child sleepy | ||
* Ask a doctor before use if you have glaucoma | * Ask a doctor before use if you have [[glaucoma]] | ||
* Trouble urinating due to an enlarged prostate gland | * Trouble [[urinating]] due to an enlarged [[prostate gland]] | ||
* A breathing problem such as emphysema or chronic bronchitis | * A breathing problem such as [[emphysema]] or [[chronic bronchitis]] | ||
* Ask a doctor or pharmacist before use if you are taking sedatives or tranquilizers | * Ask a doctor or [[pharmacist]] before use if you are taking [[sedatives]] or [[tranquilizers]] | ||
When using this product | When using this product | ||
marked drowsiness may occur | marked [[drowsiness]] may occur | ||
avoid alcoholic drinks | avoid [[alcoholic]] drinks | ||
excitability may occur, especially in children | excitability may occur, especially in children | ||
alcohol, sedatives and tranquilizers may increase drowsiness | [[alcohol]], [[sedatives]] and [[tranquilizers]] may increase [[drowsiness]] | ||
be careful when driving a motor vehicle or operating machinery | be careful when driving a motor vehicle or operating machinery | ||
If pregnant or breast-feeding, | If [[pregnant]] or [[breast-feeding]], | ||
ask a health professional before use. | ask a health professional before use. | ||
Keep out of reach of children. | Keep out of reach of children. | ||
In case of overdose, get medical help or contact a Poison Control Center right away. | In case of [[overdose]], get medical help or contact a [[Poison]] Control Center right away. | ||
|clinicalTrials | |clinicalTrials=====Digestive===== | ||
* Xerostomia | * [[Xerostomia]] | ||
=====Neurologic===== | =====Neurologic===== | ||
* Sedation | * [[Sedation]] | ||
* Dizziness | * [[Dizziness]] | ||
* Dyskinesia | * [[Dyskinesia]] | ||
* Somnolence | * [[Somnolence]] | ||
=====Respiratory===== | =====Respiratory===== | ||
* Dryness of nasal mucosa | * Dryness of [[nasal mucosa]] | ||
* Pharyngeal dryness | * [[Pharyngeal]] dryness | ||
* Thick sputum | * Thick [[sputum]] | ||
* Bronchial dryness | * [[Bronchial]] dryness | ||
=====Miscellaneous==== | =====Miscellaneous==== | ||
* Anaphylaxis reaction | * [[Anaphylaxis]] reaction | ||
<!--Postmarketing Experience--> | <!--Postmarketing Experience--> | ||
|postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of Diphenhydramine in the drug label. | |postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of Diphenhydramine in the drug label. | ||
<!--Drug Interactions--> | <!--Drug Interactions--> | ||
| Line 164: | Line 110: | ||
|useInNursing=There is no FDA guidance on the use of Diphenhydramine with respect to nursing mothers. | |useInNursing=There is no FDA guidance on the use of Diphenhydramine with respect to nursing mothers. | ||
|useInPed=* Keep out of reach of children. | |useInPed=* Keep out of reach of children. | ||
* In case of overdose, get medical help or contact a Poison Control Center right away. | * In case of overdose, get medical help or contact a [[Poison]] Control Center right away. | ||
|useInGeri=There is no FDA guidance on the use of Diphenhydramine with respect to geriatric patients. | |useInGeri=There is no FDA guidance on the use of Diphenhydramine with respect to geriatric patients. | ||
|useInGender=There is no FDA guidance on the use of Diphenhydramine with respect to specific gender populations. | |useInGender=There is no FDA guidance on the use of Diphenhydramine with respect to specific gender populations. | ||
| Line 178: | Line 124: | ||
* Oral | * Oral | ||
|monitoring=There is limited information regarding <i>Monitoring</i> of Diphenhydramine in the drug label. | |monitoring=There is limited information regarding <i>Monitoring</i> of Diphenhydramine in the drug label. | ||
<!--IV Compatibility--> | <!--IV Compatibility--> | ||
| Line 274: | Line 219: | ||
[[File:Diphenhydramine dosage table01.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | [[File:Diphenhydramine dosage table01.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
|fdaPatientInfo=* Marked drowsiness may occur | |fdaPatientInfo=* Marked drowsiness may occur | ||
* Avoid alcoholic drinks | * Avoid [[alcoholic]] drinks | ||
* Excitability may occur, especially in children | * Excitability may occur, especially in children | ||
* Alcohol, sedatives and tranquilizers may increase drowsiness | * [[Alcohol]], [[sedatives]] and [[tranquilizers]] may increase [[drowsiness]] | ||
* Be careful when driving a motor vehicle or operating machinery | * Be careful when driving a motor vehicle or operating machinery | ||
|alcohol=* Avoid alcoholic drinks. | |alcohol=* Avoid alcoholic drinks. | ||
* Alcohol, sedatives and tranquilizers may increase drowsiness. | * [[Alcohol]], [[sedatives]] and [[tranquilizers]] may increase [[drowsiness]]. | ||
|brandNames=* Benadryl® | |brandNames=* Benadryl® | ||
* Benylin Decongestant Cough® | * Benylin Decongestant Cough® | ||
| Line 299: | Line 244: | ||
{{LabelImage}} | {{LabelImage}} | ||
<!--Pill Image--> | <!--Pill Image--> | ||
<!--Label Display Image--> | <!--Label Display Image--> | ||
<!--Category--> | <!--Category--> | ||
[[Category:Drug]] | [[Category:Drug]] | ||
Revision as of 19:12, 26 February 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Adeel Jamil, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
NOTE: Most over the counter (OTC) are not reviewed and approved by the FDA. However, they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Overview
Diphenhydramine is a Analgesic and antihistamine that is FDA approved for the treatment of temporarily relieves symptoms of the common cold like Runny nose, Sneezing, Itchy watery eyes, Itchy nose or throat. Common adverse reactions include Xerostomia, dizziness, dyskinesia, sedation, somnolence, dryness of nasal mucosa, pharyngeal dryness, thick sputum..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- Temporarily relieves these symptoms of the common cold:
- Dosing Information
- Take every 4 to 6 hours, not more than 6 doses in 24 hours

Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- Chemotherapy-induced nausea and vomiting.
- Extrapyramidal disease - Medication-induced movement disorder.
- Hyperemesis gravidarum.
- Local anesthesia
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Diphenhydramine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Diphenhydramine in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Diphenhydramine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Diphenhydramine in pediatric patients.
Contraindications
- Diphenhydramine is contraindicated in persons with known hypersensitivity.
Warnings
- Do not use with any other product containing diphenhydramine, even one used on skin
- To make a child sleepy
- Ask a doctor before use if you have glaucoma
- Trouble urinating due to an enlarged prostate gland
- A breathing problem such as emphysema or chronic bronchitis
- Ask a doctor or pharmacist before use if you are taking sedatives or tranquilizers
When using this product marked drowsiness may occur avoid alcoholic drinks excitability may occur, especially in children alcohol, sedatives and tranquilizers may increase drowsiness be careful when driving a motor vehicle or operating machinery If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Adverse Reactions
Clinical Trials Experience
Digestive=
Neurologic
Respiratory
- Dryness of nasal mucosa
- Pharyngeal dryness
- Thick sputum
- Bronchial dryness
=Miscellaneous
- Anaphylaxis reaction
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Diphenhydramine in the drug label.
Drug Interactions
There is limited information regarding drug interactions of Diphenhydramine in the drug label.
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Diphenhydramine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Diphenhydramine during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Diphenhydramine with respect to nursing mothers.
Pediatric Use
- Keep out of reach of children.
- In case of overdose, get medical help or contact a Poison Control Center right away.
Geriatic Use
There is no FDA guidance on the use of Diphenhydramine with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Diphenhydramine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Diphenhydramine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Diphenhydramine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Diphenhydramine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Diphenhydramine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Diphenhydramine in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intramuscular
- Intravenous
- Oral
Monitoring
There is limited information regarding Monitoring of Diphenhydramine in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Diphenhydramine in the drug label.
Overdosage
There is limited information regarding Chronic Overdose of Diphenhydramine in the drug label.
Pharmacology
Mechanism of Action
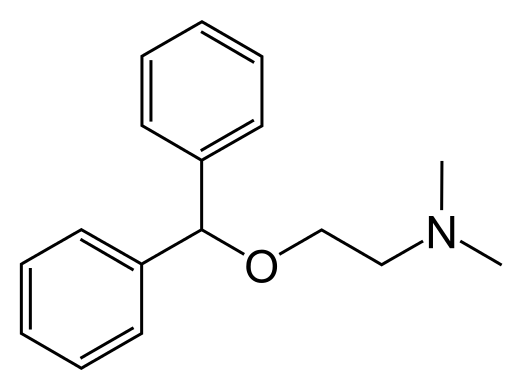
Structure

Pharmacodynamics
There is limited information regarding Pharmacodynamics of Diphenhydramine in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Diphenhydramine in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Diphenhydramine in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Diphenhydramine in the drug label.
How Supplied
Storage
store at 20°-25°C
Images
Drug Images
{{#ask: Page Name::Diphenhydramine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Diphenhydramine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Marked drowsiness may occur
- Avoid alcoholic drinks
- Excitability may occur, especially in children
- Alcohol, sedatives and tranquilizers may increase drowsiness
- Be careful when driving a motor vehicle or operating machinery
Precautions with Alcohol
- Avoid alcoholic drinks.
- Alcohol, sedatives and tranquilizers may increase drowsiness.
Brand Names
- Benadryl®
- Benylin Decongestant Cough®
- Diphenhist
- Diphenyl®
- Genahist®
- Geridryl®
- Nytol Quickcaps®
- Nytol Quickgels®
Look-Alike Drug Names
- Benadryl® - benazepril®
- diphenhydrAMINE® - dimenhyDRINATE®
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Paton DM, Webster DR (1985). "Clinical pharmacokinetics of H1-receptor antagonists (the antihistamines)". Clin. Pharmacokinet. 10 (6): 477–97. doi:10.2165/00003088-198510060-00002. PMID 2866055.
- ↑ "Showing Diphenhydramine (DB01075)". DrugBank. Retrieved 5 September 2009.
- ↑ 3.0 3.1 3.2 Simons KJ, Watson WT, Martin TJ, Chen XY, Simons FE (July 1990). "Diphenhydramine: pharmacokinetics and pharmacodynamics in elderly adults, young adults, and children". J. Clin. Pharmacol. 30 (7): 665–71. doi:10.1002/j.1552-4604.1990.tb01871.x. PMID 2391399.
- ↑ Garnett WR (February 1986). "Diphenhydramine". Am. Pharm. NS26 (2): 35–40. PMID 3962845.
{{#subobject:
|Page Name=Diphenhydramine
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Diphenhydramine |Label Name=
}}
{{#subobject:
|Label Page=Diphenhydramine |Label Name=
}}