Dimenhydrinate
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Turky Alkathery, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
NOTE: Most over the counter (OTC) are not reviewed and approved by the FDA. However, they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Overview
Dimenhydrinate is an antiemetic that is FDA approved for the treatment of these symptoms associated with motion sickness like nausea, vomiting and dizziness. Common adverse reactions include nausea, vomiting, xerostomia and sedation.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
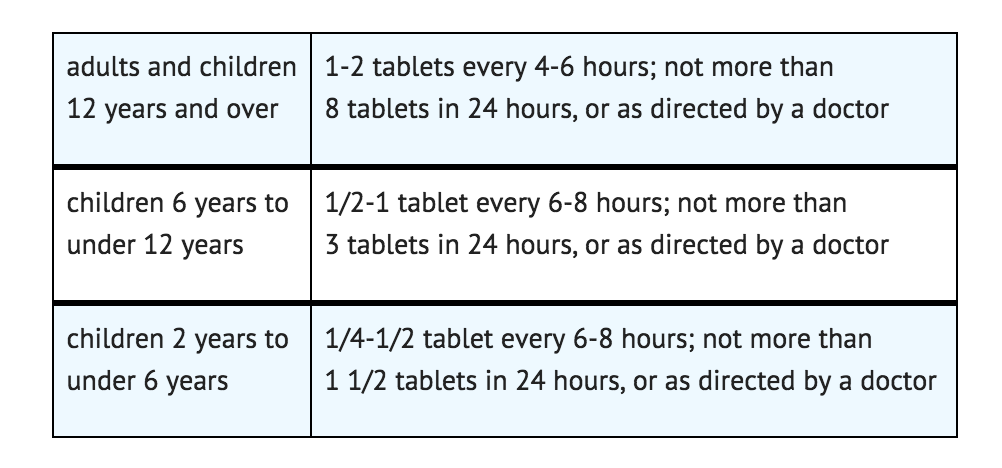
- Uses for prevention and treatment of these symptoms associated with motion sickness:
Dosage
- To prevent motion sickness, the first dose should be taken 1/2 to 1 hour before starting activity.
- To prevent or treat motion sickness, use the following dosing.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Dimenhydrinate in adult patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Dimenhydrinate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Indications
- Uses for prevention and treatment of these symptoms associated with motion sickness:
- Do not use in children under 2 years of age unless directed by a doctor
Dosage

Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Dimenhydrinate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Dimenhydrinate in pediatric patients.
Contraindications
- None
Warnings
- None.
Precautions
ASK A DOCTOR BEFORE USE IF YOU HAVE
- A breathing problem such as emphysema or chronic bronchitis.
- Glaucoma.
- Difficulty in urination due to enlargement of the prostate gland.
Adverse Reactions
Clinical Trials Experience
WHEN USING THIS PRODUCT
- Marked drowsiness may occur.
- Alcohol, sedatives and tranquilizers may increase drowsiness.
- Avoid alcoholic drinks.
- Be careful when driving a motor vehicle or operating machinery*
Postmarketing Experience
- There is limited information regarding postmarketing experience.
Drug Interactions
ASK DOCTOR/PHARMACIST
- Ask a doctor or pharmacist before use if you are taking sedatives or tranquilizers.
Use in Specific Populations
Pregnancy
- If pregnant or breast-feeding, ask a health professional before use.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Dimenhydrinate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Dimenhydrinate during labor and delivery.
Nursing Mothers
- If pregnant or breast-feeding, ask a health professional before use.
Pediatric Use
There is no FDA guidance on the use of Dimenhydrinate in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Dimenhydrinate in geriatric settings.
Gender
There is no FDA guidance on the use of Dimenhydrinate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Dimenhydrinate with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Dimenhydrinate in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Dimenhydrinate in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Dimenhydrinate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Dimenhydrinate in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral.
Monitoring
- There is limited information regarding Monitoring.
IV Compatibility
- There is limited information regarding IV Compatibility.
Overdosage
- In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222).
Pharmacology
| |
Dimenhydrinate
| |
| Combination of | |
| diphenhydramine | antiemetic |
| 8-chlorotheophylline | stimulant |
| Identifiers | |
| CAS number | |
| ATC code | R06AA52 |
| PubChem | |
| DrugBank | |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status | |
| Routes | Oral, Rectal, I.V. |
Mechanism of Action
- There is limited information regarding Mechanism of action.
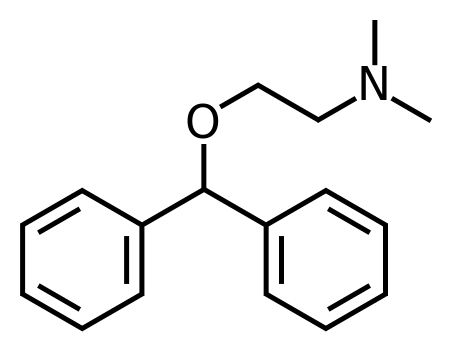
Structure

Pharmacodynamics
- There is limited information regarding Pharmacodynamics.
Pharmacokinetics
- There is limited information regarding Pharmacokinetics.
Nonclinical Toxicology
- There is limited information regarding Nonclinical toxicology.
Clinical Studies
- There is limited information regarding Clinical Studies.
How Supplied
There is limited information regarding Dimenhydrinate How Supplied in the drug label.
Storage
- Store at 15° to 30°C (59° to 86°F)
Images
Drug Images
{{#ask: Page Name::Dimenhydrinate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

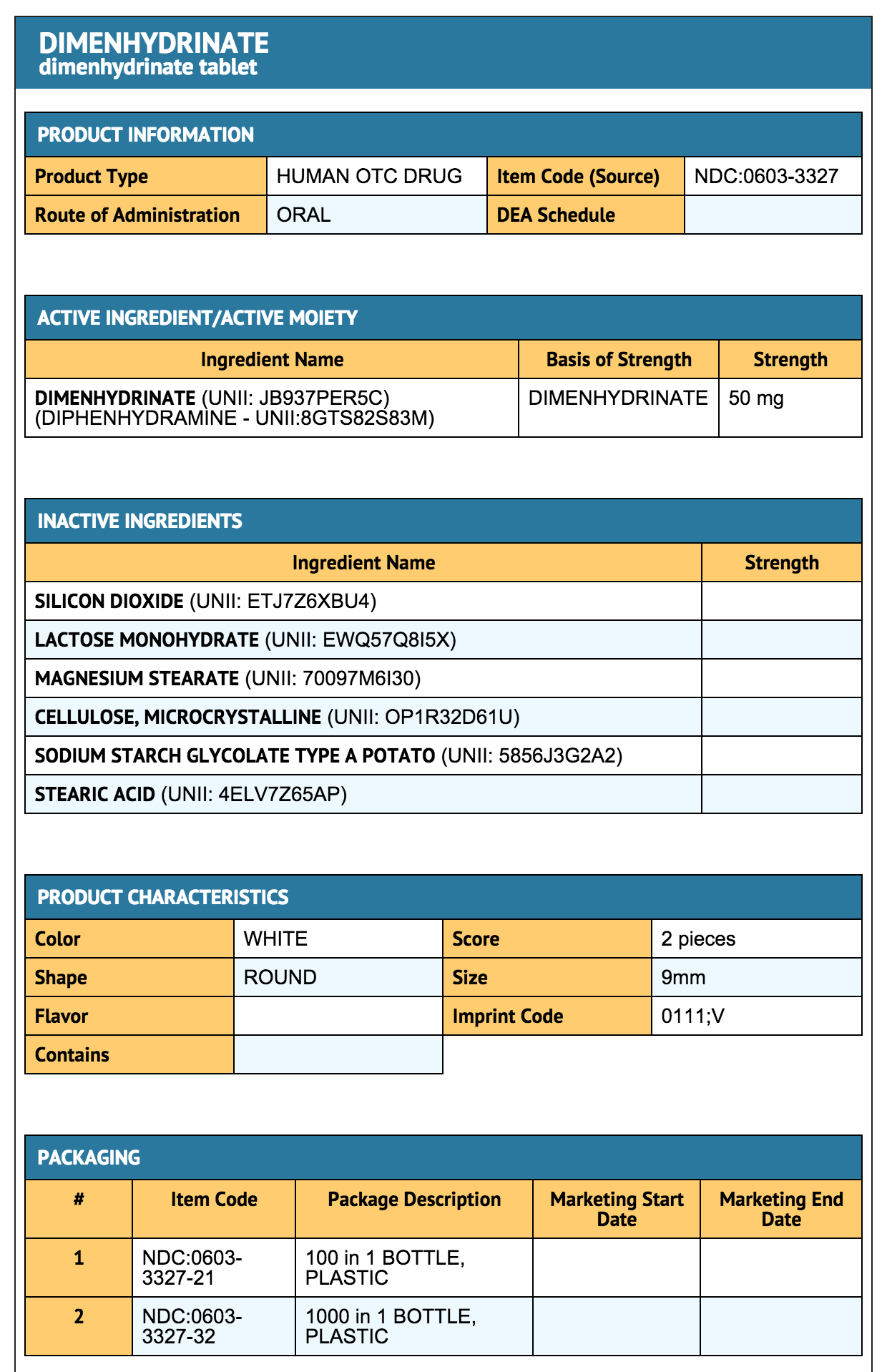
{{#ask: Label Page::Dimenhydrinate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Dimenhydrinate Patient Counseling Information in the drug label.
Precautions with Alcohol
- Avoid alcoholic drinks.
Brand Names
- DIMENHYDRINATE ®[1]
Look-Alike Drug Names
- dimenhyDRINATE - diphenhydrAMINE[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "DIMENHYDRINATE- dimenhydrinate tablet".
- ↑ "http://www.ismp.org". External link in
|title=(help)