Dihydrofolate reductase: Difference between revisions
m (Bot: Automated text replacement (-{{SIB}} + & -{{EH}} + & -{{EJ}} + & -{{Editor Help}} + & -{{Editor Join}} +)) |
m (Robot: Automated text replacement (-{{WikiDoc Cardiology Network Infobox}} +, -<references /> +{{reflist|2}}, -{{reflist}} +{{reflist|2}})) |
||
| Line 77: | Line 77: | ||
==References== | ==References== | ||
{{reflist}} | {{reflist|2}} | ||
==Further reading== | ==Further reading== | ||
{{refbegin | 2}} | {{refbegin | 2}} | ||
Revision as of 16:47, 4 September 2012
| Dihydrofolate reductase | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||
| Identifiers | |||||||||||
| Symbols | DHFR ; | ||||||||||
| External IDs | Template:OMIM5 Template:MGI HomoloGene: 56470 | ||||||||||
| EC number | 1.5.1.3 | ||||||||||
| |||||||||||
| Orthologs | |||||||||||
| Template:GNF Ortholog box | |||||||||||
| Species | Human | Mouse | |||||||||
| Entrez | n/a | n/a | |||||||||
| Ensembl | n/a | n/a | |||||||||
| UniProt | n/a | n/a | |||||||||
| RefSeq (mRNA) | n/a | n/a | |||||||||
| RefSeq (protein) | n/a | n/a | |||||||||
| Location (UCSC) | n/a | n/a | |||||||||
| PubMed search | n/a | n/a | |||||||||
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
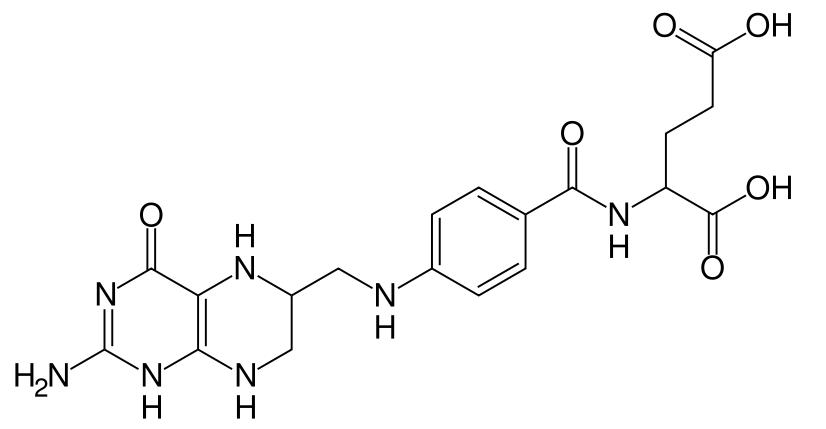
Dihydrofolate reductase, or DHFR, reduces dihydrofolic acid to tetrahydrofolic acid, using NADPH as electron donor, which can be converted to the kinds of tetrahydrofolate cofactors used in 1-carbon transfer chemistry.
Dihydrofolate reductase converts dihydrofolate into tetrahydrofolate, a methyl group shuttle required for the de novo synthesis of purines, thymidylic acid, and certain amino acids. While the functional dihydrofolate reductase gene has been mapped to chromosome 5, multiple intronless processed pseudogenes or dihydrofolate reductase-like genes have been identified on separate chromosomes. Dihydrofolate reductase deficiency has been linked to megaloblastic anemia.[1]
Clinical significance
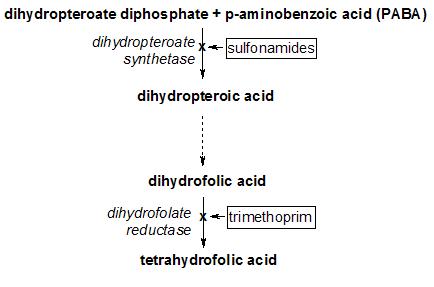
Because tetrahydrofolate, the product of this reaction, is the active form of folate in humans, inhibition of DHFR can cause functional folate deficiency. Because folate is needed by rapidly dividing cells to make thymine, this effect may be therapeutic. For example, methotrexate is used as cancer chemotherapy because it can prevent neoplastic cells from dividing.
A variety of drugs act on dihydrofolate reductase:
- the antibiotic trimethoprim.
- the antimalarial drug pyrimethamine.
- the chemotherapeutic agents methotrexate, raltitrexed and pemetrexed. Methotrexate, the first anticancer drug, acts on this enzyme binding to it some 1000 times more tightly than folate itself.
Deficiency of the enzyme may be a cause of folate deficiency, and therefore of megaloblastic anemia. Treatment is with reduced forms of folic acid.
References
Further reading
- Banerjee D, Mayer-Kuckuk P, Capiaux G; et al. (2002). "Novel aspects of resistance to drugs targeted to dihydrofolate reductase and thymidylate synthase". Biochim. Biophys. Acta. 1587 (2–3): 164–73. PMID 12084458.
- Stockman BJ, Nirmala NR, Wagner G; et al. (1992). "Sequence-specific 1H and 15N resonance assignments for human dihydrofolate reductase in solution". Biochemistry. 31 (1): 218–29. PMID 1731871.
- Beltzer JP, Spiess M (1991). "In vitro binding of the asialoglycoprotein receptor to the beta adaptin of plasma membrane coated vesicles". EMBO J. 10 (12): 3735–42. PMID 1935897.
- Davies JF, Delcamp TJ, Prendergast NJ; et al. (1991). "Crystal structures of recombinant human dihydrofolate reductase complexed with folate and 5-deazafolate". Biochemistry. 29 (40): 9467–79. PMID 2248959.
- Will CL, Dolnick BJ (1990). "5-Fluorouracil inhibits dihydrofolate reductase precursor mRNA processing and/or nuclear mRNA stability in methotrexate-resistant KB cells". J. Biol. Chem. 264 (35): 21413–21. PMID 2592384.
- Masters JN, Attardi G (1985). "Discrete human dihydrofolate reductase gene transcripts present in polysomal RNA map with their 5' ends several hundred nucleotides upstream of the main mRNA start site". Mol. Cell. Biol. 5 (3): 493–500. PMID 2859520.
- Miszta H, Dabrowski Z, Lanotte M (1988). "In vitro patterns of enzymic tetrahydrofolate dehydrogenase (EC 1.5.1.3) expression in bone marrow stromal cells". Leukemia. 2 (11): 754–9. PMID 3185016.
- Oefner C, D'Arcy A, Winkler FK (1988). "Crystal structure of human dihydrofolate reductase complexed with folate". Eur. J. Biochem. 174 (2): 377–85. PMID 3383852.
- Yang JK, Masters JN, Attardi G (1984). "Human dihydrofolate reductase gene organization. Extensive conservation of the G + C-rich 5' non-coding sequence and strong intron size divergence from homologous mammalian genes". J. Mol. Biol. 176 (2): 169–87. PMID 6235374.
- Masters JN, Yang JK, Cellini A, Attardi G (1983). "A human dihydrofolate reductase pseudogene and its relationship to the multiple forms of specific messenger RNA". J. Mol. Biol. 167 (1): 23–36. PMID 6306253.
- Chen MJ, Shimada T, Moulton AD; et al. (1984). "The functional human dihydrofolate reductase gene". J. Biol. Chem. 259 (6): 3933–43. PMID 6323448.
- Funanage VL, Myoda TT, Moses PA, Cowell HR (1985). "Assignment of the human dihydrofolate reductase gene to the q11----q22 region of chromosome 5". Mol. Cell. Biol. 4 (10): 2010–6. PMID 6504041.
- Masters JN, Attardi G (1983). "The nucleotide sequence of the cDNA coding for the human dihydrofolic acid reductase". Gene. 21 (1–2): 59–63. PMID 6687716.
- Morandi C, Masters JN, Mottes M, Attardi G (1982). "Multiple forms of human dihydrofolate reductase messenger RNA. Cloning and expression in Escherichia coli of their DNA coding sequence". J. Mol. Biol. 156 (3): 583–607. PMID 6750132.
- Bonifaci N, Sitia R, Rubartelli A (1996). "Nuclear translocation of an exogenous fusion protein containing HIV Tat requires unfolding". AIDS. 9 (9): 995–1000. PMID 8527095.
- Mayhew M, da Silva AC, Martin J; et al. (1996). "Protein folding in the central cavity of the GroEL-GroES chaperonin complex". Nature. 379 (6564): 420–6. doi:10.1038/379420a0. PMID 8559246.
- Gross M, Robinson CV, Mayhew M; et al. (1997). "Significant hydrogen exchange protection in GroEL-bound DHFR is maintained during iterative rounds of substrate cycling". Protein Sci. 5 (12): 2506–13. PMID 8976559.
- Schleiff E, Shore GC, Goping IS (1997). "Human mitochondrial import receptor, Tom20p. Use of glutathione to reveal specific interactions between Tom20-glutathione S-transferase and mitochondrial precursor proteins". FEBS Lett. 404 (2–3): 314–8. PMID 9119086.
- Cody V, Galitsky N, Luft JR; et al. (1997). "Comparison of two independent crystal structures of human dihydrofolate reductase ternary complexes reduced with nicotinamide adenine dinucleotide phosphate and the very tight-binding inhibitor PT523". Biochemistry. 36 (45): 13897–903. doi:10.1021/bi971711l. PMID 9374868.
- Vanguri VK, Wang S, Godyna S; et al. (2001). "Thrombospondin-1 binds to polyhistidine with high affinity and specificity". Biochem. J. 347 (Pt 2): 469–73. PMID 10749676.
External links
bg:Дихидрофолатредуктаза de:Dihydrofolatreduktase it:Diidrofolato reduttasi he:DHFR no:Dihydrofolat reduktase