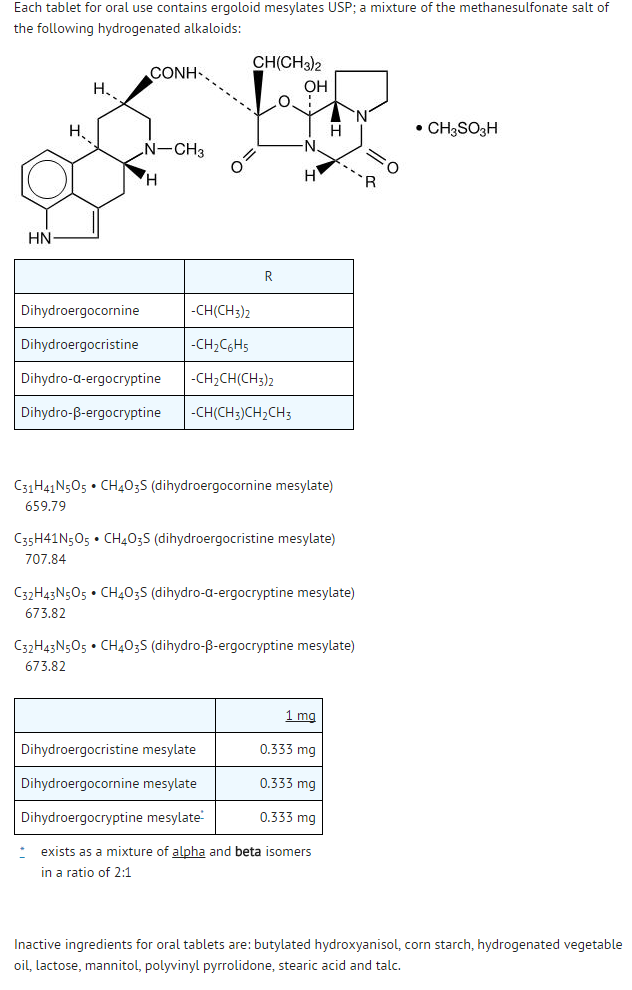
Dihydroergocristine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Dihydroergocristine is an ergot alkaloid that is FDA approved for the treatment of dementia. Common adverse reactions include flushing, rash, nausea, vomiting, headache, blurred vision, congestion of nasal sinus.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- A proportion of individuals over sixty who manifest signs and symptoms of an idiopathic decline in mental capacity (i.e., cognitive and interpersonal skills, mood, self-care, apparent motivation) can experience some symptomatic relief upon treatment with ergoloid mesylates preparations. The identity of the specific trait(s) or condition(s), if any, which would usefully predict a response to ergoloid mesylates therapy is not known. It appears, however, that those individuals who do respond come from groups of patients who would be considered clinically to suffer from some ill-defined process related to aging or to have some underlying dementing condition (i.e., primary progressive dementia, Alzheimer's dementia, senile onset, multi-infarct dementia).
- Before prescribing ergoloid mesylates, the physician should exclude the possibility that the patient's signs and symptoms arise from a potentially reversible and treatable condition. Particular care should be taken to exclude delirium and dementiform illness secondary to systemic disease, primary neurological disease, or primary disturbance of mood. Ergoloid mesylates preparations are not indicated in the treatment of acute or chronic psychosis, regardless of etiology.
- The decision to use ergoloid mesylates in the treatment of an individual with a symptomatic decline in mental capacity of unknown etiology should be continually reviewed since the presenting clinical picture may subsequently evolve sufficiently to allow a specific diagnosis and a specific alternative treatment. In addition, continued clinical evaluation is required to determine whether any initial benefit conferred by ergoloid mesylates therapy persists with time.
- The efficacy of ergoloid mesylates was evaluated using a special rating scale known as the SCAG (Sandoz Clinical Assessment-Geriatric). The specific items on this scale on which modest but statistically significant changes were observed at the end of twelve weeks include: mental alertness, confusion, recent memory, orientation, emotional lability, self-care, depression, anxiety/fears, cooperation, sociability, appetite, dizziness, fatigue, bothersome(ness), and an overall impression of clinical status.
- 1 mg three times a day.
- Alleviation of symptoms is usually gradual and results may not be observed for 3–4 weeks.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Dihydroergocristine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Dihydroergocristine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Dihydroergocristine in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Dihydroergocristine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Dihydroergocristine in pediatric patients.
Contraindications
CONTRAINDICATIONS
- Ergoloid mesylates preparations are contraindicated in individuals who have previously shown hypersensitivity to the drug. Ergoloid mesylates preparations are also contraindicated in patients who have psychosis, acute or chronic, regardless of etiology.
Warnings
- Practitioners are advised that because the target symptoms are of unknown etiology, careful diagnosis should be attempted before prescribing ergoloid mesylates preparations.
Adverse Reactions
Clinical Trials Experience
- Ergoloid mesylates preparations have not been found to produce serious side effects.
- Transient nausea and gastric disturbances have been reported. Ergoloid mesylates preparations do not possess the vasoconstrictor properties of the natural ergot alkaloids.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Dihydroergocristine in the drug label.
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Dihydroergocristine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Dihydroergocristine during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Dihydroergocristine with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Dihydroergocristine with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Dihydroergocristine with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Dihydroergocristine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Dihydroergocristine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Dihydroergocristine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Dihydroergocristine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Dihydroergocristine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Dihydroergocristine in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
There is limited information regarding Monitoring of Dihydroergocristine in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Dihydroergocristine in the drug label.
Overdosage
There is limited information regarding Chronic Overdose of Dihydroergocristine in the drug label.
Pharmacology
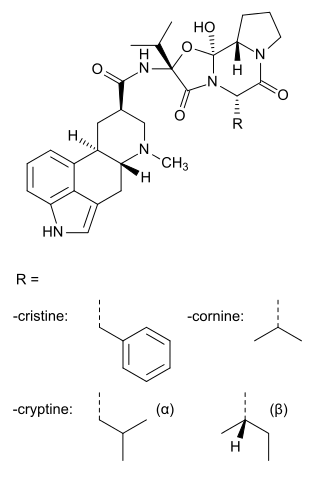
| |
Dihydroergocristine
| |
| Combination of | |
| Dihydroergocristine | Ergot alkaloid |
| Dihydroergocornine | Ergot alkaloid |
| alpha-Dihydroergocryptine | Ergot alkaloid |
| beta-Dihydroergocryptine | Ergot alkaloid |
| Identifiers | |
| CAS number | |
| ATC code | C04 |
| PubChem | ? |
| DrugBank | |
| Therapeutic considerations | |
| Pregnancy cat. |
Contraindicated |
| Legal status |
Template:Unicode Prescription only |
| Routes | Oral, parenteral |
Mechanism of Action
- There is no specific evidence which clearly establishes the mechanism by which ergoloid mesylates preparations produce mental effects, nor is there conclusive evidence that the drug particularly affects cerebral arteriosclerosis or cerebrovascular insufficiency.
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Dihydroergocristine in the drug label.
Pharmacokinetics
- Pharmacokinetic studies have been performed in normal volunteers with the help of radiolabelled drug as well as employing a specific radioimmunoassay technique. From the urinary excretion quotient of orally and intravenously administered tritium-labelled ergoloid mesylates the absorption of ergoloid was calculated to be 25%.
- Following oral administration, peak levels of 0.5 ngEq/mL/mg were achieved within 1.5–3 hr. Bioavailability studies with the specific radioimmunoassay confirm that ergoloid is rapidly absorbed from the gastrointestinal tract, with mean peak levels of 0.05–0.13 ng/mL/mg (with extremes of 0.03 and 0.18 ng/mL/mg) achieved within 0.6–1.3 hr (with extremes of 0.4 and 2.8 hr). The finding of lower peak levels of ergoloid compared to the total drug-metabolite composite is consistent with a considerable first pass liver metabolism, with less than 50% of the therapeutic moiety reaching the systemic circulation. The elimination of radioactivity, representing ergoloid plus metabolites bearing the radiolabel, was biphasic with half-lives of 4 and 13 hr. The mean half-life of unchanged ergoloid in plasma is about 2.6–5.1 hr; after 3 half-lives ergoloid plasma levels are less than 10% of radioactivity levels, and by 24 hr no ergoloid is detectable.
- Bioequivalence studies were performed comparing ergoloid mesylates oral tablets (administered orally) with ergoloid mesylates sublingual tablets (administered sublingually). The oral tablet and sublingual tablet were shown to be bioequivalent.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Dihydroergocristine in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Dihydroergocristine in the drug label.
How Supplied
- Ergoloid mesylates tablets, USP (oral) 1 mg are white, round, unscored, debossed MP 20
- Bottles of 50 NDC 53489-281-02
- Bottles of 100 NDC 53489-281-01
- Bottles of 250 NDC 53489-281-03
- Bottles of 500 NDC 53489-281-05
- Bottles of 1000 NDC 53489-281-10
Storage
Store at 20° to 25°C (68° to 77°F)
Images
Drug Images
{{#ask: Page Name::Dihydroergocristine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Dihydroergocristine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Dihydroergocristine in the drug label.
Precautions with Alcohol
- Alcohol-Dihydroergocristine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ERGOLOID MESYLATES®[1]
Look-Alike Drug Names
There is limited information regarding Dihydroergocristine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Dihydroergocristine |Label Name=Ergot mesylate 01.jpg
}}
{{#subobject:
|Label Page=Dihydroergocristine |Label Name=Ergot mesylate 02.png
}}