Dihydrodesoxymorphine: Difference between revisions
m (Protected "Dihydrodesoxymorphine": Protecting pages from unwanted edits ([edit=sysop] (indefinite) [move=sysop] (indefinite))) |
m (Robot: Automated text replacement (-{{WikiDoc Cardiology Network Infobox}} +, -<references /> +{{reflist|2}}, -{{reflist}} +{{reflist|2}})) |
||
| (One intermediate revision by the same user not shown) | |||
| Line 29: | Line 29: | ||
{{CMG}} | {{CMG}} | ||
__NOTOC__ | __NOTOC__ | ||
==Overview== | ==Overview== | ||
| Line 36: | Line 36: | ||
== References == | == References == | ||
{{reflist|2}} | |||
{{opioids}} | {{opioids}} | ||
Latest revision as of 16:47, 4 September 2012
 | |
| Clinical data | |
|---|---|
| Synonyms | Desomorphine, Dihydrodesoxymorphine, Permonid |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
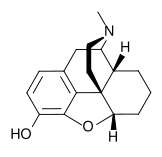
| Formula | C17H21NO2 |
| Molar mass | 271.354 g/mol |
| 3D model (JSmol) | |
| |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Desomorphine (Dihydrodesoxymorphine, Permonid) is an opiate analogue invented in 1933 in the United States, that is a derivative of morphine, where the 6-hydroxy group has been removed and the 7,8 double bond has been saturated. It has sedative and analgesic effects, and is around 10 times more potent than morphine.[1][2][3] It was used in Switzerland under the brand name Permonid, and was described as having a fast onset and a short duration of action, with relatively little nausea or respiratory depression compared to equivalent doses of morphine. This drug has attracted recent attention in Russia due to an upsurge in clandestine production, presumably due to its relatively simple synthesis from codeine.[4] It is prepared from α-chlorocodide, which is itself obtained by reacting thionyl chloride with codeine. By catalytic reduction, α-chlorocodide gives dihydrodesoxycodeine, which yields desomorphine on demethylation.[5][6]
References
- ↑ Bognar R, Makleit S. New method for the preparation of dihydro-6-desoxymorphine. (German). Arzneimittelforschung. 1958 Jun;8(6):323-5. PMID 13546093
- ↑ Janssen PA. A review of the chemical features associated with strong morphine-like activity. British Journal of Anaesthesia. 1962 Apr;34(4):260-268. PMID 14451235
- ↑ Sargent LJ, May EL. Agonists--antagonists derived from desomorphine and metopon. Journal of Medicinal Chemistry. 1970 Nov;13(6):1061-3. PMID 4098039
- ↑ Drug police RB is alarmed with growth of Desomorphine usage
- ↑ Mosettig E, Cohen FL, Small LF. Desoxycodeine Studies. III. The Constitution of the So-Called Alpha-Dehydrodesoxycodeine: Bis-Di-hydrodesoxycodeine. Journal of the American Chemical Society 1932; 54:793-801.
- ↑ Eddy NB, Howes HA. Studies of Morphine, Codeine and their Derivatives X. Desoxymorphine-C, Desoxycodeine-C and their Hydrogenated Derivatives. Journal of Pharmacology And Experimental Therapeutics. 1935; 55(3):257-267.
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs missing an ATC code
- Articles containing unverified chemical infoboxes
- Opioids