Diclofenamide: Difference between revisions
No edit summary |
No edit summary |
||
| Line 14: | Line 14: | ||
:* Recommended initial dosage: '''100-200 mg PO bid''' | :* Recommended initial dosage: '''100-200 mg PO bid''' | ||
:* Recommended maintenence dosage: '''25-50 mg PO qd or bid or tid''' | :* Recommended maintenence dosage: '''25-50 mg PO qd or bid or tid''' | ||
|contraindications=* [[Hepatic Insufficiency]] | |||
:* DARANIDE® (dichlorphenamide tablets USP) is contraindicated in [[hepatic insufficiency]]. | |||
* Electrolyte Imbalance | |||
:* DARANIDE® is contraindicated in [[renal failure]], [[adrenocortical insufficiency]], [[hyperchloremic acidosis]], or in conditions in which serum levels of sodium or potassium are depressed. | |||
* Obstructive Pulmonary Disease | |||
:* DARANIDE® should not be used in patients with severe pulmonary obstruction who are unable to increase their alveolar ventilation since their acidosis may be increased. | |||
* [[Hypersensitivity]] | |||
:* DARANIDE® is contraindicated in patients who are [[hypersensitive]] to this product. | |||
|warnings======Hypokalemia===== | |||
Potassium excretion is increased by DARANIDE® (dichlorphenamide tablets USP) and [[hypokalemia]] may develop. | |||
Interference with adequate oral electrolyte intake will also contribute to [[hypokalemia]]. [[Hypokalemia]] can sensitize or exaggerate the response of the heart to the toxic effects of digitalis (e.g., increased [[ventricular irritability]]). [[Hypokalemia]] may be avoided or treated by use of potassium supplements such as foods with a high potassium content. | |||
: | |clinicalTrials=The most common adverse reactions include gastrointestinal disturbances ([[anorexia]], [[nausea]], and [[vomiting]]), [[drowsiness]] and [[paresthesias]]. | ||
| | Certain adverse reactions characteristic of carbonic anhydrase inhibitors may result with DARANIDE® (dichlorphenamide tablets USP), particularly with increasing doses. The following are adverse reactions which have been reported with systemic carbonic anhydrase inhibitors. The pharmacological similarities among the carbonic anhydrase inhibitors make it advisable to consider the following reactions when dichlorphenamide is administered: [[agranulocytosis]], [[ataxia]], [[confusion]], [[constipation]], [[depression]], [[disorientation]], [[dizziness]], electrolyte imbalance ([[hypokalemia]], [[hyperchloremia]]), [[fever], [[globus hystericus]], [[headache]], [[hepatic insufficiency]], [[hyperuricemia]], kidney stones, [[lassitude]], [[leucopenia]], metabolic acidosis, [[nervousness]], [[phosphaturia]], [[pruritus]], [[renal colic]], skin eruptions, [[thrombocytopenia]], [[tinnitus]], [[tremor]], urinary frequency, weakness, and weight loss. | ||
|drugInteractions=* High-dose Aspirin | |||
=====High-dose Aspirin===== | |||
[[Anorexia]], [[tachypnea]], [[lethargy]] and [[coma]] have been rarely reported due to a possible drug interaction with high-dose [[aspirin]]. | |||
|FDAPregCat=C | |||
|useInPregnancyFDA=Pregnancy Category C. Dichlorphenamide has been shown to be teratogenic in the rat (skeletal anomalies) when given in doses 100 times the human dose. There are no adequate and well-controlled studies in pregnant women. DARANIDE® (dichlorphenamide tablets USP) should not be used in women of childbearing age or in pregnancy, especially during the first trimester, unless the potential benefits outweigh the potential risks. | |||
|useInPregnancyAUS=(Description) | |||
|useInNursing=It is not known whether dichlorphenamide is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when dichlorphenamide is administered to a nursing woman. | |||
|useInPed=Safety and effectiveness in pediatric patients have not been established. | |||
| | |||
(Description | |||
| | |||
| | |||
|useInGeri=Metabolic acidosis, which can be severe, may occur in the elderly with reduced renal function. | |||
|useInRenalImpair=(Description) | |useInRenalImpair=(Description) | ||
|useInHepaticImpair=(Description) | |useInHepaticImpair=(Description) | ||
Revision as of 21:06, 22 April 2014
{{DrugProjectFormSinglePage |authorTag=Sheng Shi, M.D. [1] |genericName=Diclofenamide |aOrAn=a |drugClass=Adrenergic receptor agonist |indication=elevated intraoculr pressure |adverseReactions=anorexia, nausea, vomiting, drowsiness, paresthesias. |blackBoxWarningTitle=Warning Title |blackBoxWarningBody=Condition Name: (Content) |fdaLIADAdult======Elevated Intraoculr Pressure=====
- Dosing Information
- Recommended initial dosage: 100-200 mg PO bid
- Recommended maintenence dosage: 25-50 mg PO qd or bid or tid
|contraindications=* Hepatic Insufficiency
- DARANIDE® (dichlorphenamide tablets USP) is contraindicated in hepatic insufficiency.
- Electrolyte Imbalance
- DARANIDE® is contraindicated in renal failure, adrenocortical insufficiency, hyperchloremic acidosis, or in conditions in which serum levels of sodium or potassium are depressed.
- Obstructive Pulmonary Disease
- DARANIDE® should not be used in patients with severe pulmonary obstruction who are unable to increase their alveolar ventilation since their acidosis may be increased.
- DARANIDE® is contraindicated in patients who are hypersensitive to this product.
|warnings======Hypokalemia=====
Potassium excretion is increased by DARANIDE® (dichlorphenamide tablets USP) and hypokalemia may develop. Interference with adequate oral electrolyte intake will also contribute to hypokalemia. Hypokalemia can sensitize or exaggerate the response of the heart to the toxic effects of digitalis (e.g., increased ventricular irritability). Hypokalemia may be avoided or treated by use of potassium supplements such as foods with a high potassium content.
|clinicalTrials=The most common adverse reactions include gastrointestinal disturbances (anorexia, nausea, and vomiting), drowsiness and paresthesias. Certain adverse reactions characteristic of carbonic anhydrase inhibitors may result with DARANIDE® (dichlorphenamide tablets USP), particularly with increasing doses. The following are adverse reactions which have been reported with systemic carbonic anhydrase inhibitors. The pharmacological similarities among the carbonic anhydrase inhibitors make it advisable to consider the following reactions when dichlorphenamide is administered: agranulocytosis, ataxia, confusion, constipation, depression, disorientation, dizziness, electrolyte imbalance (hypokalemia, hyperchloremia), [[fever], globus hystericus, headache, hepatic insufficiency, hyperuricemia, kidney stones, lassitude, leucopenia, metabolic acidosis, nervousness, phosphaturia, pruritus, renal colic, skin eruptions, thrombocytopenia, tinnitus, tremor, urinary frequency, weakness, and weight loss. |drugInteractions=* High-dose Aspirin
High-dose Aspirin
Anorexia, tachypnea, lethargy and coma have been rarely reported due to a possible drug interaction with high-dose aspirin. |FDAPregCat=C |useInPregnancyFDA=Pregnancy Category C. Dichlorphenamide has been shown to be teratogenic in the rat (skeletal anomalies) when given in doses 100 times the human dose. There are no adequate and well-controlled studies in pregnant women. DARANIDE® (dichlorphenamide tablets USP) should not be used in women of childbearing age or in pregnancy, especially during the first trimester, unless the potential benefits outweigh the potential risks. |useInPregnancyAUS=(Description) |useInNursing=It is not known whether dichlorphenamide is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when dichlorphenamide is administered to a nursing woman. |useInPed=Safety and effectiveness in pediatric patients have not been established.
|useInGeri=Metabolic acidosis, which can be severe, may occur in the elderly with reduced renal function.
|useInRenalImpair=(Description) |useInHepaticImpair=(Description) |useInReproPotential=(Description) |useInImmunocomp=(Description) |othersTitle=Others |useInOthers=(Description) |administration=(Oral/Intravenous/etc) |monitoring======Condition 1=====
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section) |IVCompat====Solution===
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Y-Site
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Admixture
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Syringe
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
TPN/TNA
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
|overdose====Acute Overdose===
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description) |drugBox=
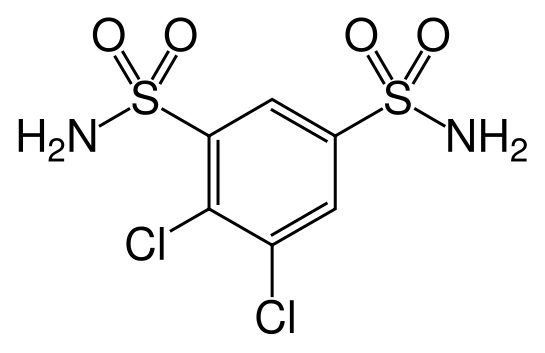
| |
Diclofenamide
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
|mechAction=(Description) |structure=(Description with picture) |PD=(Description) |PK=(Description) |nonClinToxic=(Description) |clinicalStudies======Condition 1=====
(Description)
Condition 2
(Description)
Condition 3
(Description) |howSupplied=(Description) |fdaPatientInfo=(Patient Counseling Information) |nlmPatientInfo=(Link to patient information page) |lookAlike=* (Paired Confused Name 1a) — (Paired Confused Name 1b)
- (Paired Confused Name 2a) — (Paired Confused Name 2b)
- (Paired Confused Name 3a) — (Paired Confused Name 3b)
|drugShortage=Drug Shortage }}