Diclofenac: Difference between revisions
m (Bot: Automated text replacement (-{{SIB}} + & -{{EH}} + & -{{EJ}} + & -{{Editor Help}} + & -{{Editor Join}} +)) |
No edit summary |
||
| Line 1: | Line 1: | ||
{{ | {{DrugProjectFormSinglePage | ||
|authorTag= | |||
{{VP}} | |||
<!--Overview--> | |||
|genericName= | |||
Diclofenac | |||
|aOrAn= | |||
a | |||
|drugClass= | |||
[[non-steroidal anti-inflammatory drug]] ([[NSAID]]) | |||
|indication= | |||
[[primary dysmenorrhea]], relief of mild to moderate [[pain]], relief of the signs and symptoms of [[osteoarthritis]], relief of the signs and symptoms of [[rheumatoid arthritis]] | |||
|hasBlackBoxWarning= | |||
Yes | |||
|adverseReactions= | |||
<!--Black Box Warning--> | |||
|blackBoxWarningTitle= | |||
Cardiovascular and Gastrointestinal Risk | |||
|blackBoxWarningBody= | |||
<i><span style="color:#FF0000;"> </span></i> | |||
*Cardiovascular Risk | |||
:*NSAIDs may cause an increased risk of serious cardiovascular thrombotic events, myocardial infarction and stroke, which can be fatal. This risk may increase with duration of use. Patients with cardiovascular disease or risk factors for cardiovascular disease may be at greater risk. | |||
:*Diclofenac potassium is contraindicated for the treatment of perioperative pain in the setting of coronary artery bypass graft (CABG) surgery. | |||
*Gastrointestinal Risk | |||
:* NSAIDs cause an increased risk of serious gastrointestinal adverse events including inflammation, bleeding, ulceration and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients are at greater risk for serious gastrointestinal events. | |||
<!--Adult Indications and Dosage--> | |||
<!--FDA-Labeled Indications and Dosage (Adult)--> | |||
|fdaLIADAdult= | |||
=====Primary Dysmenorrhea===== | |||
* Dosing Information | |||
:* The recommended dosage is 50 mg t.i.d. With experience, physicians may find that in some patients an initial dose of 100 mg of diclofenac potassium tablets, followed by 50 mg doses, will provide better relief. | |||
=====Mild to moderate pain===== | |||
* Dosing Information | |||
:* The recommended dosage is 50 mg t.i.d. With experience, physicians may find that in some patients an initial dose of 100 mg of diclofenac potassium tablets, followed by 50 mg doses, will provide better relief. | |||
=====Relief Of Osteoarthritis===== | |||
* Dosing Information | |||
:*100 to 150 mg/day in divided doses, 50 mg b.i.d. or t.i.d. | |||
=====Relief of Rheumatoid arthritis===== | |||
* Dosing Information | |||
:*150 to 200 mg/day in divided doses, 50 mg t.i.d. or q.i.d. | |||
<!--Off-Label Use and Dosage (Adult)--> | |||
<!--Guideline-Supported Use (Adult)--> | |||
|offLabelAdultGuideSupport= | |||
=====Condition1===== | |||
* Developed by: | |||
* Class of Recommendation: | |||
* Strength of Evidence: | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Non–Guideline-Supported Use (Adult)--> | |||
|offLabelAdultNoGuideSupport= | |||
=====Condition1===== | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Pediatric Indications and Dosage--> | |||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | |||
|fdaLIADPed= | |||
=====Condition1===== | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Off-Label Use and Dosage (Pediatric)--> | |||
<!--Guideline-Supported Use (Pediatric)--> | |||
|offLabelPedGuideSupport= | |||
=====Condition1===== | |||
* Developed by: | |||
* Class of Recommendation: | |||
* Strength of Evidence: | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Non–Guideline-Supported Use (Pediatric)--> | |||
|offLabelPedNoGuideSupport= | |||
=====Condition1===== | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Contraindications--> | |||
|contraindications= | |||
*Diclofenac potassium tablets are contraindicated in patients with known [[hypersensitivity]] to diclofenac. | |||
*Diclofenac potassium should not be given to patients who have experienced [[asthma]], [[urticaria]] or allergic type reactions after taking [[aspirin]] or other [[NSAIDs]]. Severe, rarely fatal, anaphylactic-like reactions to [[NSAIDs]] have been reported in such patients. | |||
*Diclofenac potassium is contraindicated for the treatment of [[perioperative pain]] in the setting of [[coronary artery bypass graft]] (CABG) surgery. | |||
<!--Warnings--> | |||
|warnings= | |||
=====Cardiovascular Effects===== | |||
*Cardiovascular Thrombotic Events | |||
:*Clinical trials of several COX-2 selective and nonselective NSAIDs of up to 3 years duration have shown an increased risk of serious cardiovascular (CV) thrombotic events, myocardial infarction and stroke, which can be fatal. All NSAIDs, both COX-2 selective and nonselective, may have a similar risk. Patients with known CV disease or risk factors for CV disease may be at greater risk. To minimize the potential risk for an adverse CV event in patients treated with an NSAID, the lowest effective dose should be used for the shortest duration possible. Physicians and patients should remain alert for the development of such events, even in the absence of previous CV symptoms. Patients should be informed about the signs and/or symptoms of serious CV events and the steps to take if they occur. | |||
:*There is no consistent evidence that concurrent use of aspirin mitigates the increased risk of serious CV thrombotic events associated with NSAID use. The concurrent use of aspirin and an NSAID does increase the risk of serious GI events (see WARNINGS: Gastrointestinal (GI) Effects). | |||
:*Two large, controlled, clinical trials of a COX-2 selective NSAID for the treatment of pain in the first 10 to 14 days following CABG surgery found an increased incidence of myocardial infarction and stroke (see CONTRAINDICATIONS). | |||
*Hypertension | |||
:*NSAIDs can lead to onset of new hypertension or worsening of preexisting hypertension, either of which may contribute to the increased incidence of CV events. Patients taking thiazides or loop diuretics may have impaired response to these therapies when taking NSAIDs. NSAIDs, including diclofenac potassium, should be used with caution in patients with hypertension. Blood pressure (BP) should be monitored closely during the initiation of NSAID treatment and throughout the course of therapy. | |||
*Congestive Heart Failure and Edema | |||
:*Fluid retention and edema have been observed in some patients taking NSAIDs. Diclofenac potassium should be used with caution in patients with fluid retention or heart failure. | |||
=====Gastrointestinal (GI) Effects===== | |||
*Risk of GI Ulceration, Bleeding and Perforation | |||
:*NSAIDs, including diclofenac potassium, can cause serious gastrointestinal (GI) adverse events including inflammation, bleeding, ulceration and perforation of the stomach, small intestine or large intestine, which can be fatal. These serious adverse events can occur at any time, with or without warning symptoms, in patients treated with NSAIDs. Only one in five patients, who develop a serious upper GI adverse event on NSAID therapy, is symptomatic. Upper GI ulcers, gross bleeding or perforation caused by NSAIDs occur in approximately 1% of patients treated for 3 to 6 months, and in about 2% to 4% of patients treated for one year. These trends continue with longer duration of use, increasing the likelihood of developing a serious GI event at some time during the course of therapy. However, even short-term therapy is not without risk. | |||
:*NSAIDs should be prescribed with extreme caution in those with a prior history of ulcer disease or gastrointestinal bleeding. Patients with a prior history of peptic ulcer disease and/or gastrointestinal bleeding who use NSAIDs have a greater than 10-fold increased risk for developing a GI bleed compared to patients with neither of these risk factors. Other factors that increase the risk for GI bleeding in patients treated with NSAIDs include concomitant use of oral corticosteroids or anticoagulants, longer duration of NSAID therapy, smoking, use of alcohol, older age and poor general health status. Most spontaneous reports of fatal GI events are in elderly or debilitated patients and therefore special care should be taken in treating this population. | |||
:*To minimize the potential risk for an adverse GI event in patients treated with an NSAID, the lowest effective dose should be used for the shortest possible duration. Patients and physicians should remain alert for signs and symptoms of GI ulceration and bleeding during NSAID therapy and promptly initiate additional evaluation and treatment if a serious GI adverse event is suspected. This should include discontinuation of the NSAID until a serious GI adverse event is ruled out. For high risk patients, alternate therapies that do not involve NSAIDs should be considered. | |||
=====Renal Effects===== | |||
*Caution should be used when initiating treatment with diclofenac potassium in patients with considerable dehydration. | |||
*Long-term administration of NSAIDs has resulted in renal papillary necrosis and other renal injury. Renal toxicity has also been seen in patients in whom renal prostaglandins have a compensatory role in the maintenance of renal perfusion. In these patients, administration of a non-steroidal anti-inflammatory drug may cause a dose dependent reduction in prostaglandin formation and, secondarily, in renal blood flow, which may precipitate overt renal decompensation. Patients at greatest risk of this reaction are those with impaired renal function, heart failure, liver dysfunction, those taking diuretics and ACE inhibitors and the elderly. Discontinuation of NSAID therapy is usually followed by recovery to the pretreatment state. | |||
*Advanced Renal Disease | |||
:*No information is available from controlled clinical studies regarding the use of diclofenac potassium in patients with advanced renal disease. Therefore, treatment with diclofenac potassium is not recommended in these patients with advanced renal disease. If diclofenac potassium therapy must be initiated, close monitoring of the patient’s renal function is advisable. | |||
=====Hepatic Effects===== | |||
*Elevations of one or more liver tests may occur during therapy with diclofenac potassium. These laboratory abnormalities may progress, may remain unchanged or may be transient with continued therapy. Borderline elevations (i.e., less than 3 times the ULN [ULN = the upper limit of the normal range]) or greater elevations of transaminases occurred in about 15% of diclofenac-treated patients. Of the markers of hepatic function, ALT (SGPT) is recommended for the monitoring of liver injury. | |||
*In clinical trials, meaningful elevations (i.e., more than 3 times the ULN) of AST (GOT) (ALT was not measured in all studies) occurred in about 2% of approximately 5,700 patients at some time during diclofenac treatment. In a large, open-label, controlled trial of 3,700 patients treated for 2 to 6 months, patients were monitored first at 8 weeks and 1,200 patients were monitored again at 24 weeks. Meaningful elevations of ALT and/or AST occurred in about 4% of patients and included marked elevations (i.e., more than 8 times the ULN) in about 1% of the 3,700 patients. In that open-label study, a higher incidence of borderline (less than 3 times the ULN), moderate (3 to 8 times the ULN), and marked (> 8 times the ULN) elevations of ALT or AST was observed in patients receiving diclofenac when compared to other NSAIDs. Elevations in transaminases were seen more frequently in patients with osteoarthritis than in those with rheumatoid arthritis. | |||
*Almost all meaningful elevations in transaminases were detected before patients became symptomatic. Abnormal tests occurred during the first 2 months of therapy with diclofenac in 42 of the 51 patients in all trials who developed marked transaminase elevations. | |||
*In post-marketing reports, cases of drug-induced hepatotoxicity have been reported in the first month, and in some cases, the first 2 months of therapy, but can occur at any time during treatment with diclofenac. Post-marketing surveillance has reported cases of severe hepatic reactions, including liver necrosis, jaundice, fulminant hepatitis with and without jaundice and liver failure. Some of these reported cases resulted in fatalities or liver transplantation. | |||
*Physicians should measure transaminases periodically in patients receiving long-term therapy with diclofenac, because severe hepatotoxicity may develop without a prodrome of distinguishing symptoms. The optimum times for making the first and subsequent transaminase measurements are not known. Based on clinical trial data and post-marketing experiences, transaminases should be monitored within 4 to 8 weeks after initiating treatment with diclofenac. However, severe hepatic reactions can occur at any time during treatment with diclofenac. | |||
*If abnormal liver tests persist or worsen, if clinical signs and/or symptoms consistent with liver disease develop or if systemic manifestations occur (e.g., eosinophilia, rash, abdominal pain, diarrhea, dark urine, etc.), diclofenac potassium should be discontinued immediately. | |||
*To minimize the possibility that hepatic injury will become severe between transaminase measurements, physicians should inform patients of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, diarrhea, pruritus, jaundice, right upper quadrant tenderness and "flu-like" symptoms), and the appropriate action patients should take if these signs and symptoms appear. | |||
*To minimize the potential risk for an adverse liver related event in patients treated with diclofenac potassium, the lowest effective dose should be used for the shortest duration possible. Caution should be exercised in prescribing diclofenac potassium with concomitant drugs that are known to be potentially hepatotoxic (e.g., antibiotics, anti-epileptics). | |||
=====Anaphylactoid Reactions===== | |||
*As with other NSAIDs, anaphylactic reactions may occur both in patients with the aspirin triad and in patients without known sensitivity to NSAIDs or known prior exposure to diclofenac potassium. Diclofenac potassium should not be given to patients with the aspirin triad. This symptom complex typically occurs in asthmatic patients who experience rhinitis with or without nasal polyps, or who exhibit severe, potentially fatal bronchospasm after taking aspirin or other NSAIDs (see CONTRAINDICATIONS and PRECAUTIONS: General: Preexisting Asthma). Anaphylaxis-type reactions have been reported with NSAID products, including with diclofenac products, such as diclofenac potassium. Emergency help should be sought in cases where an anaphylactic reaction occurs. | |||
=====Skin Reactions===== | |||
*NSAIDs, including diclofenac potassium, can cause serious skin adverse events such as exfoliative dermatitis, Stevens-Johnson Syndrome (SJS) and toxic epidermal necrolysis (TEN), which can be fatal. These serious events may occur without warning. Patients should be informed about the signs and symptoms of serious skin manifestations and use of the drug should be discontinued at the first appearance of skin rash or any other sign of hypersensitivity. | |||
=====Pregnancy===== | |||
*In late pregnancy, as with other NSAIDs, diclofenac potassium should be avoided because it may cause premature closure of the ductus arteriosus. | |||
====Precautions==== | |||
*General | |||
:*Diclofenac potassium cannot be expected to substitute for corticosteroids or to treat corticosteroid insufficiency. Abrupt discontinuation of corticosteroids may lead to disease exacerbation. Patients on prolonged corticosteroid therapy should have their therapy tapered slowly if a decision is made to discontinue corticosteroids. | |||
:*The pharmacological activity of diclofenac potassium in reducing fever and inflammation may diminish the utility of these diagnostic signs in detecting complications of presumed noninfectious, painful conditions. | |||
*Hematological Effects | |||
:*Anemia is sometimes seen in patients receiving NSAIDs, including diclofenac potassium. This may be due to fluid retention, occult or gross GI blood loss or an incompletely described effect upon erythropoiesis. Patients on long-term treatment with NSAIDs, including diclofenac potassium, should have their hemoglobin or hematocrit checked if they exhibit any signs or symptoms of anemia. | |||
:*NSAIDs inhibit platelet aggregation and have been shown to prolong bleeding time in some patients. Unlike aspirin, their effect on platelet function is quantitatively less, of shorter duration, and reversible. Patients receiving diclofenac potassium who may be adversely affected by alterations in platelet function, such as those with coagulation disorders or patients receiving anticoagulants, should be carefully monitored. | |||
*Preexisting Asthma | |||
:*Patients with asthma may have aspirin-sensitive asthma. The use of aspirin in patients with aspirin-sensitive asthma has been associated with severe bronchospasm which can be fatal. Since cross-reactivity, including bronchospasm, between aspirin and other non-steroidal anti-inflammatory drugs has been reported in such aspirin-sensitive patients, diclofenac potassium should not be administered to patients with this form of aspirin-sensitivity and should be used with caution in all patients with preexisting asthma. | |||
<!--Adverse Reactions--> | |||
<!--Clinical Trials Experience--> | |||
|clinicalTrials= | |||
*In 718 patients treated for shorter periods, i.e., 2 weeks or less, with diclofenac potassium tablets, adverse reactions were reported one-half to one-tenth as frequently as by patients treated for longer periods. In a 6-month, double-blind trial comparing diclofenac potassium tablets (N = 196) vs. diclofenac sodium delayed-release tablets (N = 197) vs. ibuprofen (N = 197), adverse reactions were similar in nature and frequency. | |||
*In patients taking diclofenac potassium or other NSAIDs, the most frequently reported adverse experiences occurring in approximately 1% to 10% of patients are: | |||
*Gastrointestinal experiences including: abdominal pain, constipation, diarrhea, dyspepsia, flatulence, gross bleeding/perforation, heartburn, nausea, GI ulcers (gastric/duodenal) and vomiting. | |||
*Abnormal renal function, anemia, dizziness, edema, elevated liver enzymes, headaches, increased bleeding time, pruritus, rashes and tinnitus. | |||
*Additional adverse experiences reported occasionally include: | |||
=====Body as a Whole===== | |||
Fever, infection, sepsis | |||
=====Cardiovascular===== | |||
Congestive heart failure, hypertension, tachycardia, syncope | |||
=====Digestive===== | |||
Dry mouth, esophagitis, gastric/peptic ulcers, gastritis, gastrointestinal bleeding, glossitis, hematemesis, hepatitis, jaundice | |||
=====Hematologic and Lymphatic===== | |||
Ecchymosis, eosinophilia, leukopenia, melena, purpura, rectal bleeding, stomatitis, thrombocytopenia | |||
=====Metabolic and Nutritional===== | |||
Weight changes | |||
=====Neurologic===== | |||
Anxiety, asthenia, confusion, depression, dream abnormalities, drowsiness, insomnia, malaise, nervousness, paresthesia, somnolence, tremors, vertigo | |||
=====Respiratory===== | |||
Asthma, dyspnea | |||
=====Skin and Hypersensitivy Reactions===== | |||
Alopecia, photosensitivity, sweating increased | |||
=====Special Senses===== | |||
Blurred vision | |||
=====Urogenital===== | |||
Cystitis, dysuria, hematuria, interstitial nephritis, oliguria/polyuria, proteinuria, renal failure. | |||
*Other adverse reactions, which occur rarely are: | |||
=====Body as a Whole===== | |||
Anaphylactic reactions, appetite changes, death | |||
=====Cardiovascular===== | |||
Arrhythmia, hypotension, myocardial infarction, palpitations, vasculitis | |||
=====Digestive===== | |||
Colitis, eructation, fulminant hepatitis with and without jaundice, liver failure, liver necrosis, pancreatitis | |||
=====Hematologic and Lymphatic===== | |||
Agranulocytosis, hemolytic anemia, aplastic anemia, lymphadenopathy, pancytopenia | |||
=====Metabolic and Nutritional===== | |||
Hyperglycemia | |||
=====Neurologic===== | |||
Convulsions, coma, hallucinations, meningitis | |||
=====Respiratory===== | |||
Respiratory depression, pneumonia | |||
=====Skin and Hypersensitivy Reactions===== | |||
Angioedema, toxic epidermal necrolysis, erythema multiforme, exfoliative dermatitis, Stevens-Johnson Syndrome, urticaria | |||
=====Special Senses===== | |||
Conjunctivitis, hearing impairment. | |||
<!--Postmarketing Experience--> | |||
|postmarketing= | |||
There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |||
<!--Drug Interactions--> | |||
|drugInteractions= | |||
*Drug Interactions | |||
*Aspirin | |||
:*When diclofenac potassium is administered with aspirin, its protein binding is reduced. The clinical significance of this interaction is not known; however, as with other NSAIDs, concomitant administration of diclofenac and aspirin is not generally recommended because of the potential of increased adverse effects. | |||
*Methotrexate | |||
:*NSAIDs have been reported to competitively inhibit methotrexate accumulation in rabbit kidney slices. This may indicate that they could enhance the toxicity of methotrexate. Caution should be used when NSAIDs are administered concomitantly with methotrexate. | |||
*Cyclosporine | |||
:*Diclofenac potassium, like other NSAIDs, may affect renal prostaglandins and increase the toxicity of certain drugs. Therefore, concomitant therapy with diclofenac potassium may increase cyclosporine’s nephrotoxicity. Caution should be used when diclofenac potassium is administered concomitantly with cyclosporine. | |||
*ACE Inhibitors | |||
:*Reports suggest that NSAIDs may diminish the antihypertensive effect of ACE inhibitors. This interaction should be given consideration in patients taking NSAIDs concomitantly with ACE inhibitors. | |||
*Furosemide | |||
:*Clinical studies, as well as post-marketing observations, have shown that diclofenac potassium can reduce the natriuretic effect of furosemide and thiazides in some patients. This response has been attributed to inhibition of renal prostaglandin synthesis. During concomitant therapy with NSAIDs, the patient should be observed closely for signs of renal failure (see WARNINGS: Renal Effects), as well as to assure diuretic efficacy. | |||
*Lithium | |||
:*NSAIDs have produced an elevation of plasma lithium levels and a reduction in renal lithium clearance. The mean minimum lithium concentration increased 15% and the renal clearance was decreased by approximately 20%. These effects have been attributed to inhibition of renal prostaglandin synthesis by the NSAID. Thus, when NSAIDs and lithium are administered concurrently, subjects should be observed carefully for signs of lithium toxicity. | |||
*Warfarin | |||
:*The effects of warfarin and NSAIDs on GI bleeding are synergistic, such that users of both drugs together have a risk of serious GI bleeding higher than users of either drug alone. | |||
*CYP2C9 Inhibitors or Inducers | |||
:*Diclofenac is metabolized by cytochrome P450 enzymes, predominantly by CYP2C9. Coadministration of diclofenac with CYP2C9 inhibitors (e.g. voriconazole) may enhance the exposure and toxicity of diclofenac whereas coadministration with CYP2C9 inducers (e.g. rifampin) may lead to compromised efficacy of diclofenac. Use caution when dosing diclofenac with CYP2C9 inhibitors or inducers, a dosage adjustment may be warranted. | |||
<!--Use in Specific Populations--> | |||
|useInPregnancyFDA= | |||
* '''Pregnancy Category C''' | |||
*Reproductive studies conducted in rats and rabbits have not demonstrated evidence of developmental abnormalities. However, animal reproduction studies are not always predictive of human response. There are no adequate and well controlled studies in pregnant women. | |||
*Nonteratogenic Effects | |||
:*Because of the known effects of non-steroidal anti-inflammatory drugs on the fetal cardiovascular system (closure of ductus arteriosus), use during pregnancy (particularly late pregnancy) should be avoided. | |||
|useInPregnancyAUS= | |||
* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | |||
|useInLaborDelivery= | |||
*In rat studies with NSAIDs, as with other drugs known to inhibit prostaglandin synthesis, an increased incidence of dystocia, delayed parturition and decreased pup survival occurred. The effects of diclofenac potassium on labor and delivery in pregnant women are unknown. | |||
|useInNursing= | |||
*It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from diclofenac potassium, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. | |||
|useInPed= | |||
*Safety and effectiveness in pediatric patients have not been established. | |||
|useInGeri= | |||
*As with any NSAIDs, caution should be exercised in treating the elderly (65 years and older). | |||
|useInGender= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |||
|useInRace= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInRenalImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | |||
|useInHepaticImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | |||
|useInReproPotential= | |||
There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |||
|useInImmunocomp= | |||
There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
<!--Administration and Monitoring--> | |||
|administration= | |||
* Oral | |||
* Intramuscular | |||
|monitoring= | |||
There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |||
<!--IV Compatibility--> | |||
|IVCompat= | |||
There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
<!--Overdosage--> | |||
|overdose= | |||
===Acute Overdose=== | |||
====Signs and Symptoms==== | |||
*Symptoms following acute NSAID overdoses are usually limited to lethargy, drowsiness, nausea, vomiting and epigastric pain, which are generally reversible with supportive care. Gastrointestinal bleeding can occur. Hypertension, acute renal failure, respiratory depression and coma may occur, but are rare. Anaphylactoid reactions have been reported with therapeutic ingestion of NSAIDs, and may occur following an overdose. | |||
====Management==== | |||
*Patients should be managed by symptomatic and supportive care following an NSAID overdose. There are no specific antidotes. Emesis and/or activated charcoal (60 g to 100 g in adults, 1 to 2 g/kg in children) and/or osmotic cathartic may be indicated in patients seen within 4 hours of ingestion with symptoms or following a large overdose (5 to 10 times the usual dose). Forced diuresis, alkalinization of urine, hemodialysis or hemoperfusion may not be useful due to high protein binding. | |||
===Chronic Overdose=== | |||
There is limited information regarding <i>Chronic Overdose</i> of {{PAGENAME}} in the drug label. | |||
<!--Pharmacology--> | |||
<!--Drug box 2--> | |||
|drugBox= | |||
{{drugbox2 | Verifiedfields = changed | |||
| verifiedrevid = 443636249 | |||
| IUPAC_name = 2-(2-(2,6-dichlorophenylamino)phenyl)acetic acid | | IUPAC_name = 2-(2-(2,6-dichlorophenylamino)phenyl)acetic acid | ||
| image = Diclofenac.png | | image = Diclofenac.png | ||
| width= | | width = 200px | ||
| image2 = | |||
| width2 = | |||
<!--Clinical data--> | |||
| tradename = Aclonac, Cataflam, Voltaren | |||
| Drugs.com = {{drugs.com|monograph|diclofenac-epolamine}} | |||
| MedlinePlus = a689002 | |||
| pregnancy_AU = C | |||
| pregnancy_category = C (1st. and 2nd. trimester), D (third trimester)<small>([[United States|US]])</small> | |||
| legal_AU = S2-S4 | |||
| legal_UK = P | |||
| legal_status = Rx-only most preparations/countries, limited OTC in some countries, manufacture and veterinary use is banned in India, Nepal, and Pakistan due to imminent extinction of local vultures | |||
| routes_of_administration = oral, rectal, [[intramuscular]], [[intravenous]] (renal- and gallstones), topical | |||
<!--Pharmacokinetic data--> | |||
| protein_bound = More than 99% | |||
| metabolism = hepatic, no active metabolites exist | |||
| elimination_half-life = 1.2-2 hr (35% of the drug enters enterohepatic recirculation) | |||
| excretion = 40% biliary 60% urine | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 15307-86-5 | | CAS_number = 15307-86-5 | ||
| ATC_prefix = | | ATC_prefix = D11 | ||
| ATC_suffix = | | ATC_suffix = AX18 | ||
| ATC_supplemental = {{ATC|M02|AA15}}, {{ATC|S01|BC03}} | | ATC_supplemental = {{ATC|M01|AB05}}, {{ATC|M02|AA15}}, {{ATC|S01|BC03}} | ||
| PubChem = 3033 | | PubChem = 3033 | ||
| DrugBank = | | IUPHAR_ligand = 2714 | ||
| C=14 |H=11 |Cl=2 |N=1 |O=2 | | DrugBank_Ref = {{drugbankcite|changed|drugbank}} | ||
| DrugBank = DB00586 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 2925 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = 144O8QL0L1 | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D07816 | |||
| ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ChEBI = 47381 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 139 | |||
| PDB_ligand = DIF | |||
<!--Chemical data--> | |||
| C=14 | H=11 | Cl=2 | N=1 | O=2 | |||
| molecular_weight = 296.148 g/mol | | molecular_weight = 296.148 g/mol | ||
| | | smiles = O=C(O)Cc1ccccc1Nc2c(Cl)cccc2Cl.OCCN1CCCC1 | ||
| InChI = 1/C14H11Cl2NO2.C6H13NO/c15-10-5-3-6-11(16)14(10)17-12-7-2-1-4-9(12)8-13(18)19;8-6-5-7-3-1-2-4-7/h1-7,17H,8H2,(H,18,19);8H,1-6H2 | |||
| | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| StdInChI = 1S/C14H11Cl2NO2/c15-10-5-3-6-11(16)14(10)17-12-7-2-1-4-9(12)8-13(18)19/h1-7,17H,8H2,(H,18,19) | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| | | StdInChIKey = DCOPUUMXTXDBNB-UHFFFAOYSA-N | ||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
}} | }} | ||
<!--Mechanism of Action--> | |||
|mechAction= | |||
*Diclofenac potassium is a [[non-steroidal anti-inflammatory drug]] ([[NSAID]]) that exhibits anti-inflammatory, analgesic and antipyretic activities in animal models. The mechanism of action of diclofenac potassium, like that of other NSAIDs, is not completely understood but may be related to [[prostaglandin]] synthetase inhibition. | |||
<!--Structure--> | |||
|structure= | |||
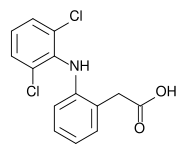
* Diclofenac, as the potassium salt, is a benzeneacetic acid derivative, designated chemically as 2-[(2,6-dichlorophenyl)amino]benzeneacetic acid, monopotassium salt. The structural formula, molecular formula and molecular weight are as follows: | |||
: [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
*Diclofenac, USP, as the potassium salt, is a faintly yellowish white to light beige, virtually odorless, slightly hygroscopic crystalline powder. It is freely soluble in methanol, soluble in ethanol, and practically insoluble in chloroform and in dilute acid. Diclofenac potassium is soluble in water. The n-octanol/water partition coefficient is 13.4 at pH 7.4 and 1545 at pH 5.2. It has a single dissociation constant (pKa) of 4 ± 0.2 at 25°C in water. | |||
*Diclofenac potassium tablets, USP for oral administration, contain 50 mg of diclofenac potassium, USP. In addition, each tablet contains the following inactive ingredients: anhydrous lactose, colloidal silicon dioxide, croscarmellose sodium, glyceryl triacetate, hypromellose, magnesium stearate, microcrystalline cellulose, polydextrose, polyethylene glycol, sodium lauryl sulfate and titanium dioxide. | |||
<!--Pharmacodynamics--> | |||
|PD= | |||
*Diclofenac potassium is a non-steroidal anti-inflammatory drug (NSAID) that exhibits anti-inflammatory, analgesic and antipyretic activities in animal models. The mechanism of action of diclofenac potassium, like that of other NSAIDs, is not completely understood but may be related to prostaglandin synthetase inhibition. | |||
<!--Pharmacokinetics--> | |||
|PK= | |||
*Absorption | |||
:*Diclofenac is 100% absorbed after oral administration compared to IV administration as measured by urine recovery. However, due to first-pass metabolism, only about 50% of the absorbed dose is systemically available (see Table 1). In some fasting volunteers, measurable plasma levels are observed within 10 minutes of dosing with diclofenac potassium. Peak plasma levels are achieved approximately one hour in fasting normal volunteers, with a range of .33 to 2 hours. Food has no significant effect on the extent of diclofenac absorption. However, there is usually a delay in the onset of absorption and a reduction in peak plasma levels of approximately 30%. | |||
*Distribution | |||
The | :*The apparent volume of distribution (V/F) of diclofenac potassium is 1.3 L/kg. | ||
:*Diclofenac is more than 99% bound to human serum proteins, primarily to albumin. Serum protein binding is constant over the concentration range (0.15 to 105 mcg/mL) achieved with recommended doses. | |||
:*Diclofenac diffuses into and out of the synovial fluid. Diffusion into the joint occurs when plasma levels are higher than those in the synovial fluid, after which the process reverses and synovial fluid levels are higher than plasma levels. It is not known whether diffusion into the joint plays a role in the effectiveness of diclofenac. | |||
The | *Metabolism | ||
:*Five diclofenac metabolites have been identified in human plasma and urine. The metabolites include 4'-hydroxy-, 5-hydroxy-, 3'-hydroxy-, 4', 5-dihydroxy- and 3'-hydroxy-4'-methoxy diclofenac. The major diclofenac metabolite, 4'-hydroxy-diclofenac, has very weak pharmacologic activity. The formation of 4’-hydroxy-diclofenac is primarily mediated by CPY2C9. Both diclofenac and its oxidative metabolites undergo glucuronidation or sulfation followed by biliary excretion. Acylglucuronidation mediated by UGT2B7 and oxidation mediated by CPY2C8 may also play a role in diclofenac metabolism. CYP3A4 is responsible for the formation of minor metabolites, 5-hydroxy- and 3’-hydroxy-diclofenac. In patients with renal dysfunction, peak concentrations of metabolites 4'-hydroxy- and 5-hydroxy-diclofenac were approximately 50% and 4% of the parent compound after single oral dosing compared to 27% and 1% in normal healthy subjects. | |||
*Excretion | |||
:*Diclofenac is eliminated through metabolism and subsequent urinary and biliary excretion of the glucuronide and the sulfate conjugates of the metabolites. Little or no free unchanged diclofenac is excreted in the urine. Approximately 65% of the dose is excreted in the urine and approximately 35% in the bile as conjugates of unchanged diclofenac plus metabolites. Because renal elimination is not a significant pathway of elimination for unchanged diclofenac, dosing adjustment in patients with mild to moderate renal dysfunction is not necessary. The terminal half-life of unchanged diclofenac is approximately 2 hours. | |||
*Drug Interactions | |||
:*When coadministered with voriconazole (inhibitor of CYP2C9, 2C19 and 3A4 enzyme), the Cmax and AUC of diclofenac increased by 114% and 78%, respectively. | |||
*Special Populations | |||
*Pediatric | |||
:*The pharmacokinetics of diclofenac potassium has not been investigated in pediatric patients. | |||
*Race | |||
:*Pharmacokinetic differences due to race have not been identified. | |||
*Hepatic Insufficiency | |||
:*Hepatic metabolism accounts for almost 100% of diclofenac potassium elimination, so patients with hepatic disease may require reduced doses of diclofenac potassium compared to patients with normal hepatic function. | |||
*Renal Insufficiency | |||
:*Diclofenac pharmacokinetics has been investigated in subjects with renal insufficiency. No differences in the pharmacokinetics of diclofenac have been detected in studies of patients with renal impairment. In patients with renal impairment (inulin clearance 60 to 90, 30 to 60, and < 30 mL/min; N = 6 in each group), AUC values and elimination rate were comparable to those in healthy subjects. | |||
<!--Nonclinical Toxicology--> | |||
|nonClinToxic= | |||
There is limited information regarding <i>Nonclinical Toxicology</i> of {{PAGENAME}} in the drug label. | |||
<!--Clinical Studies--> | |||
= | |clinicalStudies= | ||
There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | |||
<!--How Supplied--> | |||
|howSupplied= | |||
Diclofenac | * Diclofenac Potassium Tablets, USP are available containing 50 mg of diclofenac potassium, USP. | ||
*The 50 mg tablets are white film-coated, round, unscored tablets debossed with M over D5 on one side of the tablet and blank on the other side. They are available as follows: | |||
* | :*NDC 0378-2474-01 | ||
:*bottles of 100 tablets | |||
* | |||
* | |||
:*NDC 0378-2474-05 | |||
* | :*bottles of 500 tablets | ||
*Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.] | |||
* | |||
*Protect from moisture. | |||
* | |||
*Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure. | |||
* | |||
<!--Patient Counseling Information--> | |||
= | |fdaPatientInfo= | ||
There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | |||
<!--Precautions with Alcohol--> | |||
|alcohol= | |||
* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
<!--Brand Names--> | |||
|brandNames= | |||
* DICLOFENAC POTASSIUM®<ref>{{Cite web | title = DICLOFENAC POTASSIUM- diclofenac potassium tablet, film coated | url = http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fac1f8e7-f2b4-4de3-bd5c-d798d12273c1 }}</ref> | |||
<!--Look-Alike Drug Names--> | |||
= | |lookAlike= | ||
== | * A® — B®<ref name="www.ismp.org">{{Cite web | last = | first = | title = http://www.ismp.org | url = http://www.ismp.org | publisher = | date = }}</ref> | ||
<!--Drug Shortage Status--> | |||
|drugShortage= | |||
}} | |||
<!--Pill Image--> | |||
{{PillImage | |||
|fileName=No image.jpg|This image is provided by the National Library of Medicine. | |||
|drugName= | |||
|NDC= | |||
|drugAuthor= | |||
|ingredients= | |||
|pillImprint= | |||
|dosageValue= | |||
|dosageUnit= | |||
|pillColor= | |||
|pillShape= | |||
|pillSize= | |||
|pillScore= | |||
}} | |||
<!--Label Display Image--> | |||
{{LabelImage | |||
|fileName={{PAGENAME}}11.png|This image is provided by the National Library of Medicine. | |||
}} | |||
{{LabelImage | |||
|fileName={{PAGENAME}}11.png|This image is provided by the National Library of Medicine. | |||
}} | |||
<!--Category--> | |||
[[Category:Drug]] | |||
Revision as of 19:30, 17 September 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Cardiovascular and Gastrointestinal Risk
See full prescribing information for complete Boxed Warning.
|
Overview
Diclofenac is a non-steroidal anti-inflammatory drug (NSAID) that is FDA approved for the {{{indicationType}}} of primary dysmenorrhea, relief of mild to moderate pain, relief of the signs and symptoms of osteoarthritis, relief of the signs and symptoms of rheumatoid arthritis. There is a Black Box Warning for this drug as shown here. Common adverse reactions include .
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Primary Dysmenorrhea
- Dosing Information
- The recommended dosage is 50 mg t.i.d. With experience, physicians may find that in some patients an initial dose of 100 mg of diclofenac potassium tablets, followed by 50 mg doses, will provide better relief.
Mild to moderate pain
- Dosing Information
- The recommended dosage is 50 mg t.i.d. With experience, physicians may find that in some patients an initial dose of 100 mg of diclofenac potassium tablets, followed by 50 mg doses, will provide better relief.
Relief Of Osteoarthritis
- Dosing Information
- 100 to 150 mg/day in divided doses, 50 mg b.i.d. or t.i.d.
Relief of Rheumatoid arthritis
- Dosing Information
- 150 to 200 mg/day in divided doses, 50 mg t.i.d. or q.i.d.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Diclofenac in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Diclofenac in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Diclofenac in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Diclofenac in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Diclofenac in pediatric patients.
Contraindications
- Diclofenac potassium tablets are contraindicated in patients with known hypersensitivity to diclofenac.
- Diclofenac potassium should not be given to patients who have experienced asthma, urticaria or allergic type reactions after taking aspirin or other NSAIDs. Severe, rarely fatal, anaphylactic-like reactions to NSAIDs have been reported in such patients.
- Diclofenac potassium is contraindicated for the treatment of perioperative pain in the setting of coronary artery bypass graft (CABG) surgery.
Warnings
|
Cardiovascular and Gastrointestinal Risk
See full prescribing information for complete Boxed Warning.
|
Cardiovascular Effects
- Cardiovascular Thrombotic Events
- Clinical trials of several COX-2 selective and nonselective NSAIDs of up to 3 years duration have shown an increased risk of serious cardiovascular (CV) thrombotic events, myocardial infarction and stroke, which can be fatal. All NSAIDs, both COX-2 selective and nonselective, may have a similar risk. Patients with known CV disease or risk factors for CV disease may be at greater risk. To minimize the potential risk for an adverse CV event in patients treated with an NSAID, the lowest effective dose should be used for the shortest duration possible. Physicians and patients should remain alert for the development of such events, even in the absence of previous CV symptoms. Patients should be informed about the signs and/or symptoms of serious CV events and the steps to take if they occur.
- There is no consistent evidence that concurrent use of aspirin mitigates the increased risk of serious CV thrombotic events associated with NSAID use. The concurrent use of aspirin and an NSAID does increase the risk of serious GI events (see WARNINGS: Gastrointestinal (GI) Effects).
- Two large, controlled, clinical trials of a COX-2 selective NSAID for the treatment of pain in the first 10 to 14 days following CABG surgery found an increased incidence of myocardial infarction and stroke (see CONTRAINDICATIONS).
- Hypertension
- NSAIDs can lead to onset of new hypertension or worsening of preexisting hypertension, either of which may contribute to the increased incidence of CV events. Patients taking thiazides or loop diuretics may have impaired response to these therapies when taking NSAIDs. NSAIDs, including diclofenac potassium, should be used with caution in patients with hypertension. Blood pressure (BP) should be monitored closely during the initiation of NSAID treatment and throughout the course of therapy.
- Congestive Heart Failure and Edema
- Fluid retention and edema have been observed in some patients taking NSAIDs. Diclofenac potassium should be used with caution in patients with fluid retention or heart failure.
Gastrointestinal (GI) Effects
- Risk of GI Ulceration, Bleeding and Perforation
- NSAIDs, including diclofenac potassium, can cause serious gastrointestinal (GI) adverse events including inflammation, bleeding, ulceration and perforation of the stomach, small intestine or large intestine, which can be fatal. These serious adverse events can occur at any time, with or without warning symptoms, in patients treated with NSAIDs. Only one in five patients, who develop a serious upper GI adverse event on NSAID therapy, is symptomatic. Upper GI ulcers, gross bleeding or perforation caused by NSAIDs occur in approximately 1% of patients treated for 3 to 6 months, and in about 2% to 4% of patients treated for one year. These trends continue with longer duration of use, increasing the likelihood of developing a serious GI event at some time during the course of therapy. However, even short-term therapy is not without risk.
- NSAIDs should be prescribed with extreme caution in those with a prior history of ulcer disease or gastrointestinal bleeding. Patients with a prior history of peptic ulcer disease and/or gastrointestinal bleeding who use NSAIDs have a greater than 10-fold increased risk for developing a GI bleed compared to patients with neither of these risk factors. Other factors that increase the risk for GI bleeding in patients treated with NSAIDs include concomitant use of oral corticosteroids or anticoagulants, longer duration of NSAID therapy, smoking, use of alcohol, older age and poor general health status. Most spontaneous reports of fatal GI events are in elderly or debilitated patients and therefore special care should be taken in treating this population.
- To minimize the potential risk for an adverse GI event in patients treated with an NSAID, the lowest effective dose should be used for the shortest possible duration. Patients and physicians should remain alert for signs and symptoms of GI ulceration and bleeding during NSAID therapy and promptly initiate additional evaluation and treatment if a serious GI adverse event is suspected. This should include discontinuation of the NSAID until a serious GI adverse event is ruled out. For high risk patients, alternate therapies that do not involve NSAIDs should be considered.
Renal Effects
- Caution should be used when initiating treatment with diclofenac potassium in patients with considerable dehydration.
- Long-term administration of NSAIDs has resulted in renal papillary necrosis and other renal injury. Renal toxicity has also been seen in patients in whom renal prostaglandins have a compensatory role in the maintenance of renal perfusion. In these patients, administration of a non-steroidal anti-inflammatory drug may cause a dose dependent reduction in prostaglandin formation and, secondarily, in renal blood flow, which may precipitate overt renal decompensation. Patients at greatest risk of this reaction are those with impaired renal function, heart failure, liver dysfunction, those taking diuretics and ACE inhibitors and the elderly. Discontinuation of NSAID therapy is usually followed by recovery to the pretreatment state.
- Advanced Renal Disease
- No information is available from controlled clinical studies regarding the use of diclofenac potassium in patients with advanced renal disease. Therefore, treatment with diclofenac potassium is not recommended in these patients with advanced renal disease. If diclofenac potassium therapy must be initiated, close monitoring of the patient’s renal function is advisable.
Hepatic Effects
- Elevations of one or more liver tests may occur during therapy with diclofenac potassium. These laboratory abnormalities may progress, may remain unchanged or may be transient with continued therapy. Borderline elevations (i.e., less than 3 times the ULN [ULN = the upper limit of the normal range]) or greater elevations of transaminases occurred in about 15% of diclofenac-treated patients. Of the markers of hepatic function, ALT (SGPT) is recommended for the monitoring of liver injury.
- In clinical trials, meaningful elevations (i.e., more than 3 times the ULN) of AST (GOT) (ALT was not measured in all studies) occurred in about 2% of approximately 5,700 patients at some time during diclofenac treatment. In a large, open-label, controlled trial of 3,700 patients treated for 2 to 6 months, patients were monitored first at 8 weeks and 1,200 patients were monitored again at 24 weeks. Meaningful elevations of ALT and/or AST occurred in about 4% of patients and included marked elevations (i.e., more than 8 times the ULN) in about 1% of the 3,700 patients. In that open-label study, a higher incidence of borderline (less than 3 times the ULN), moderate (3 to 8 times the ULN), and marked (> 8 times the ULN) elevations of ALT or AST was observed in patients receiving diclofenac when compared to other NSAIDs. Elevations in transaminases were seen more frequently in patients with osteoarthritis than in those with rheumatoid arthritis.
- Almost all meaningful elevations in transaminases were detected before patients became symptomatic. Abnormal tests occurred during the first 2 months of therapy with diclofenac in 42 of the 51 patients in all trials who developed marked transaminase elevations.
- In post-marketing reports, cases of drug-induced hepatotoxicity have been reported in the first month, and in some cases, the first 2 months of therapy, but can occur at any time during treatment with diclofenac. Post-marketing surveillance has reported cases of severe hepatic reactions, including liver necrosis, jaundice, fulminant hepatitis with and without jaundice and liver failure. Some of these reported cases resulted in fatalities or liver transplantation.
- Physicians should measure transaminases periodically in patients receiving long-term therapy with diclofenac, because severe hepatotoxicity may develop without a prodrome of distinguishing symptoms. The optimum times for making the first and subsequent transaminase measurements are not known. Based on clinical trial data and post-marketing experiences, transaminases should be monitored within 4 to 8 weeks after initiating treatment with diclofenac. However, severe hepatic reactions can occur at any time during treatment with diclofenac.
- If abnormal liver tests persist or worsen, if clinical signs and/or symptoms consistent with liver disease develop or if systemic manifestations occur (e.g., eosinophilia, rash, abdominal pain, diarrhea, dark urine, etc.), diclofenac potassium should be discontinued immediately.
- To minimize the possibility that hepatic injury will become severe between transaminase measurements, physicians should inform patients of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, diarrhea, pruritus, jaundice, right upper quadrant tenderness and "flu-like" symptoms), and the appropriate action patients should take if these signs and symptoms appear.
- To minimize the potential risk for an adverse liver related event in patients treated with diclofenac potassium, the lowest effective dose should be used for the shortest duration possible. Caution should be exercised in prescribing diclofenac potassium with concomitant drugs that are known to be potentially hepatotoxic (e.g., antibiotics, anti-epileptics).
Anaphylactoid Reactions
- As with other NSAIDs, anaphylactic reactions may occur both in patients with the aspirin triad and in patients without known sensitivity to NSAIDs or known prior exposure to diclofenac potassium. Diclofenac potassium should not be given to patients with the aspirin triad. This symptom complex typically occurs in asthmatic patients who experience rhinitis with or without nasal polyps, or who exhibit severe, potentially fatal bronchospasm after taking aspirin or other NSAIDs (see CONTRAINDICATIONS and PRECAUTIONS: General: Preexisting Asthma). Anaphylaxis-type reactions have been reported with NSAID products, including with diclofenac products, such as diclofenac potassium. Emergency help should be sought in cases where an anaphylactic reaction occurs.
Skin Reactions
- NSAIDs, including diclofenac potassium, can cause serious skin adverse events such as exfoliative dermatitis, Stevens-Johnson Syndrome (SJS) and toxic epidermal necrolysis (TEN), which can be fatal. These serious events may occur without warning. Patients should be informed about the signs and symptoms of serious skin manifestations and use of the drug should be discontinued at the first appearance of skin rash or any other sign of hypersensitivity.
Pregnancy
- In late pregnancy, as with other NSAIDs, diclofenac potassium should be avoided because it may cause premature closure of the ductus arteriosus.
Precautions
- General
- Diclofenac potassium cannot be expected to substitute for corticosteroids or to treat corticosteroid insufficiency. Abrupt discontinuation of corticosteroids may lead to disease exacerbation. Patients on prolonged corticosteroid therapy should have their therapy tapered slowly if a decision is made to discontinue corticosteroids.
- The pharmacological activity of diclofenac potassium in reducing fever and inflammation may diminish the utility of these diagnostic signs in detecting complications of presumed noninfectious, painful conditions.
- Hematological Effects
- Anemia is sometimes seen in patients receiving NSAIDs, including diclofenac potassium. This may be due to fluid retention, occult or gross GI blood loss or an incompletely described effect upon erythropoiesis. Patients on long-term treatment with NSAIDs, including diclofenac potassium, should have their hemoglobin or hematocrit checked if they exhibit any signs or symptoms of anemia.
- NSAIDs inhibit platelet aggregation and have been shown to prolong bleeding time in some patients. Unlike aspirin, their effect on platelet function is quantitatively less, of shorter duration, and reversible. Patients receiving diclofenac potassium who may be adversely affected by alterations in platelet function, such as those with coagulation disorders or patients receiving anticoagulants, should be carefully monitored.
- Preexisting Asthma
- Patients with asthma may have aspirin-sensitive asthma. The use of aspirin in patients with aspirin-sensitive asthma has been associated with severe bronchospasm which can be fatal. Since cross-reactivity, including bronchospasm, between aspirin and other non-steroidal anti-inflammatory drugs has been reported in such aspirin-sensitive patients, diclofenac potassium should not be administered to patients with this form of aspirin-sensitivity and should be used with caution in all patients with preexisting asthma.
Adverse Reactions
Clinical Trials Experience
- In 718 patients treated for shorter periods, i.e., 2 weeks or less, with diclofenac potassium tablets, adverse reactions were reported one-half to one-tenth as frequently as by patients treated for longer periods. In a 6-month, double-blind trial comparing diclofenac potassium tablets (N = 196) vs. diclofenac sodium delayed-release tablets (N = 197) vs. ibuprofen (N = 197), adverse reactions were similar in nature and frequency.
- In patients taking diclofenac potassium or other NSAIDs, the most frequently reported adverse experiences occurring in approximately 1% to 10% of patients are:
- Gastrointestinal experiences including: abdominal pain, constipation, diarrhea, dyspepsia, flatulence, gross bleeding/perforation, heartburn, nausea, GI ulcers (gastric/duodenal) and vomiting.
- Abnormal renal function, anemia, dizziness, edema, elevated liver enzymes, headaches, increased bleeding time, pruritus, rashes and tinnitus.
- Additional adverse experiences reported occasionally include:
Body as a Whole
Fever, infection, sepsis
Cardiovascular
Congestive heart failure, hypertension, tachycardia, syncope
Digestive
Dry mouth, esophagitis, gastric/peptic ulcers, gastritis, gastrointestinal bleeding, glossitis, hematemesis, hepatitis, jaundice
Hematologic and Lymphatic
Ecchymosis, eosinophilia, leukopenia, melena, purpura, rectal bleeding, stomatitis, thrombocytopenia
Metabolic and Nutritional
Weight changes
Neurologic
Anxiety, asthenia, confusion, depression, dream abnormalities, drowsiness, insomnia, malaise, nervousness, paresthesia, somnolence, tremors, vertigo
Respiratory
Asthma, dyspnea
Skin and Hypersensitivy Reactions
Alopecia, photosensitivity, sweating increased
Special Senses
Blurred vision
Urogenital
Cystitis, dysuria, hematuria, interstitial nephritis, oliguria/polyuria, proteinuria, renal failure.
- Other adverse reactions, which occur rarely are:
Body as a Whole
Anaphylactic reactions, appetite changes, death
Cardiovascular
Arrhythmia, hypotension, myocardial infarction, palpitations, vasculitis
Digestive
Colitis, eructation, fulminant hepatitis with and without jaundice, liver failure, liver necrosis, pancreatitis
Hematologic and Lymphatic
Agranulocytosis, hemolytic anemia, aplastic anemia, lymphadenopathy, pancytopenia
Metabolic and Nutritional
Hyperglycemia
Neurologic
Convulsions, coma, hallucinations, meningitis
Respiratory
Respiratory depression, pneumonia
Skin and Hypersensitivy Reactions
Angioedema, toxic epidermal necrolysis, erythema multiforme, exfoliative dermatitis, Stevens-Johnson Syndrome, urticaria
Special Senses
Conjunctivitis, hearing impairment.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Diclofenac in the drug label.
Drug Interactions
- Drug Interactions
- Aspirin
- When diclofenac potassium is administered with aspirin, its protein binding is reduced. The clinical significance of this interaction is not known; however, as with other NSAIDs, concomitant administration of diclofenac and aspirin is not generally recommended because of the potential of increased adverse effects.
- Methotrexate
- NSAIDs have been reported to competitively inhibit methotrexate accumulation in rabbit kidney slices. This may indicate that they could enhance the toxicity of methotrexate. Caution should be used when NSAIDs are administered concomitantly with methotrexate.
- Cyclosporine
- Diclofenac potassium, like other NSAIDs, may affect renal prostaglandins and increase the toxicity of certain drugs. Therefore, concomitant therapy with diclofenac potassium may increase cyclosporine’s nephrotoxicity. Caution should be used when diclofenac potassium is administered concomitantly with cyclosporine.
- ACE Inhibitors
- Reports suggest that NSAIDs may diminish the antihypertensive effect of ACE inhibitors. This interaction should be given consideration in patients taking NSAIDs concomitantly with ACE inhibitors.
- Furosemide
- Clinical studies, as well as post-marketing observations, have shown that diclofenac potassium can reduce the natriuretic effect of furosemide and thiazides in some patients. This response has been attributed to inhibition of renal prostaglandin synthesis. During concomitant therapy with NSAIDs, the patient should be observed closely for signs of renal failure (see WARNINGS: Renal Effects), as well as to assure diuretic efficacy.
- Lithium
- NSAIDs have produced an elevation of plasma lithium levels and a reduction in renal lithium clearance. The mean minimum lithium concentration increased 15% and the renal clearance was decreased by approximately 20%. These effects have been attributed to inhibition of renal prostaglandin synthesis by the NSAID. Thus, when NSAIDs and lithium are administered concurrently, subjects should be observed carefully for signs of lithium toxicity.
- Warfarin
- The effects of warfarin and NSAIDs on GI bleeding are synergistic, such that users of both drugs together have a risk of serious GI bleeding higher than users of either drug alone.
- CYP2C9 Inhibitors or Inducers
- Diclofenac is metabolized by cytochrome P450 enzymes, predominantly by CYP2C9. Coadministration of diclofenac with CYP2C9 inhibitors (e.g. voriconazole) may enhance the exposure and toxicity of diclofenac whereas coadministration with CYP2C9 inducers (e.g. rifampin) may lead to compromised efficacy of diclofenac. Use caution when dosing diclofenac with CYP2C9 inhibitors or inducers, a dosage adjustment may be warranted.
Use in Specific Populations
Pregnancy
- Pregnancy Category C
- Reproductive studies conducted in rats and rabbits have not demonstrated evidence of developmental abnormalities. However, animal reproduction studies are not always predictive of human response. There are no adequate and well controlled studies in pregnant women.
- Nonteratogenic Effects
- Because of the known effects of non-steroidal anti-inflammatory drugs on the fetal cardiovascular system (closure of ductus arteriosus), use during pregnancy (particularly late pregnancy) should be avoided.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Diclofenac in women who are pregnant.
Labor and Delivery
- In rat studies with NSAIDs, as with other drugs known to inhibit prostaglandin synthesis, an increased incidence of dystocia, delayed parturition and decreased pup survival occurred. The effects of diclofenac potassium on labor and delivery in pregnant women are unknown.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from diclofenac potassium, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
- As with any NSAIDs, caution should be exercised in treating the elderly (65 years and older).
Gender
There is no FDA guidance on the use of Diclofenac with respect to specific gender populations.
Race
There is no FDA guidance on the use of Diclofenac with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Diclofenac in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Diclofenac in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Diclofenac in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Diclofenac in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intramuscular
Monitoring
There is limited information regarding Monitoring of Diclofenac in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Diclofenac in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Symptoms following acute NSAID overdoses are usually limited to lethargy, drowsiness, nausea, vomiting and epigastric pain, which are generally reversible with supportive care. Gastrointestinal bleeding can occur. Hypertension, acute renal failure, respiratory depression and coma may occur, but are rare. Anaphylactoid reactions have been reported with therapeutic ingestion of NSAIDs, and may occur following an overdose.
Management
- Patients should be managed by symptomatic and supportive care following an NSAID overdose. There are no specific antidotes. Emesis and/or activated charcoal (60 g to 100 g in adults, 1 to 2 g/kg in children) and/or osmotic cathartic may be indicated in patients seen within 4 hours of ingestion with symptoms or following a large overdose (5 to 10 times the usual dose). Forced diuresis, alkalinization of urine, hemodialysis or hemoperfusion may not be useful due to high protein binding.
Chronic Overdose
There is limited information regarding Chronic Overdose of Diclofenac in the drug label.
Pharmacology
Mechanism of Action
- Diclofenac potassium is a non-steroidal anti-inflammatory drug (NSAID) that exhibits anti-inflammatory, analgesic and antipyretic activities in animal models. The mechanism of action of diclofenac potassium, like that of other NSAIDs, is not completely understood but may be related to prostaglandin synthetase inhibition.
Structure
- Diclofenac, as the potassium salt, is a benzeneacetic acid derivative, designated chemically as 2-[(2,6-dichlorophenyl)amino]benzeneacetic acid, monopotassium salt. The structural formula, molecular formula and molecular weight are as follows:
- Diclofenac, USP, as the potassium salt, is a faintly yellowish white to light beige, virtually odorless, slightly hygroscopic crystalline powder. It is freely soluble in methanol, soluble in ethanol, and practically insoluble in chloroform and in dilute acid. Diclofenac potassium is soluble in water. The n-octanol/water partition coefficient is 13.4 at pH 7.4 and 1545 at pH 5.2. It has a single dissociation constant (pKa) of 4 ± 0.2 at 25°C in water.
- Diclofenac potassium tablets, USP for oral administration, contain 50 mg of diclofenac potassium, USP. In addition, each tablet contains the following inactive ingredients: anhydrous lactose, colloidal silicon dioxide, croscarmellose sodium, glyceryl triacetate, hypromellose, magnesium stearate, microcrystalline cellulose, polydextrose, polyethylene glycol, sodium lauryl sulfate and titanium dioxide.
Pharmacodynamics
- Diclofenac potassium is a non-steroidal anti-inflammatory drug (NSAID) that exhibits anti-inflammatory, analgesic and antipyretic activities in animal models. The mechanism of action of diclofenac potassium, like that of other NSAIDs, is not completely understood but may be related to prostaglandin synthetase inhibition.
Pharmacokinetics
- Absorption
- Diclofenac is 100% absorbed after oral administration compared to IV administration as measured by urine recovery. However, due to first-pass metabolism, only about 50% of the absorbed dose is systemically available (see Table 1). In some fasting volunteers, measurable plasma levels are observed within 10 minutes of dosing with diclofenac potassium. Peak plasma levels are achieved approximately one hour in fasting normal volunteers, with a range of .33 to 2 hours. Food has no significant effect on the extent of diclofenac absorption. However, there is usually a delay in the onset of absorption and a reduction in peak plasma levels of approximately 30%.
- Distribution
- The apparent volume of distribution (V/F) of diclofenac potassium is 1.3 L/kg.
- Diclofenac is more than 99% bound to human serum proteins, primarily to albumin. Serum protein binding is constant over the concentration range (0.15 to 105 mcg/mL) achieved with recommended doses.
- Diclofenac diffuses into and out of the synovial fluid. Diffusion into the joint occurs when plasma levels are higher than those in the synovial fluid, after which the process reverses and synovial fluid levels are higher than plasma levels. It is not known whether diffusion into the joint plays a role in the effectiveness of diclofenac.
- Metabolism
- Five diclofenac metabolites have been identified in human plasma and urine. The metabolites include 4'-hydroxy-, 5-hydroxy-, 3'-hydroxy-, 4', 5-dihydroxy- and 3'-hydroxy-4'-methoxy diclofenac. The major diclofenac metabolite, 4'-hydroxy-diclofenac, has very weak pharmacologic activity. The formation of 4’-hydroxy-diclofenac is primarily mediated by CPY2C9. Both diclofenac and its oxidative metabolites undergo glucuronidation or sulfation followed by biliary excretion. Acylglucuronidation mediated by UGT2B7 and oxidation mediated by CPY2C8 may also play a role in diclofenac metabolism. CYP3A4 is responsible for the formation of minor metabolites, 5-hydroxy- and 3’-hydroxy-diclofenac. In patients with renal dysfunction, peak concentrations of metabolites 4'-hydroxy- and 5-hydroxy-diclofenac were approximately 50% and 4% of the parent compound after single oral dosing compared to 27% and 1% in normal healthy subjects.
- Excretion
- Diclofenac is eliminated through metabolism and subsequent urinary and biliary excretion of the glucuronide and the sulfate conjugates of the metabolites. Little or no free unchanged diclofenac is excreted in the urine. Approximately 65% of the dose is excreted in the urine and approximately 35% in the bile as conjugates of unchanged diclofenac plus metabolites. Because renal elimination is not a significant pathway of elimination for unchanged diclofenac, dosing adjustment in patients with mild to moderate renal dysfunction is not necessary. The terminal half-life of unchanged diclofenac is approximately 2 hours.
- Drug Interactions
- When coadministered with voriconazole (inhibitor of CYP2C9, 2C19 and 3A4 enzyme), the Cmax and AUC of diclofenac increased by 114% and 78%, respectively.
- Special Populations
- Pediatric
- The pharmacokinetics of diclofenac potassium has not been investigated in pediatric patients.
- Race
- Pharmacokinetic differences due to race have not been identified.
- Hepatic Insufficiency
- Hepatic metabolism accounts for almost 100% of diclofenac potassium elimination, so patients with hepatic disease may require reduced doses of diclofenac potassium compared to patients with normal hepatic function.
- Renal Insufficiency
- Diclofenac pharmacokinetics has been investigated in subjects with renal insufficiency. No differences in the pharmacokinetics of diclofenac have been detected in studies of patients with renal impairment. In patients with renal impairment (inulin clearance 60 to 90, 30 to 60, and < 30 mL/min; N = 6 in each group), AUC values and elimination rate were comparable to those in healthy subjects.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Diclofenac in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Diclofenac in the drug label.
How Supplied
- Diclofenac Potassium Tablets, USP are available containing 50 mg of diclofenac potassium, USP.
- The 50 mg tablets are white film-coated, round, unscored tablets debossed with M over D5 on one side of the tablet and blank on the other side. They are available as follows:
- NDC 0378-2474-01
- bottles of 100 tablets
- NDC 0378-2474-05
- bottles of 500 tablets
- Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
- Protect from moisture.
- Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
Storage
There is limited information regarding Diclofenac Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Diclofenac |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Diclofenac |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Diclofenac in the drug label.
Precautions with Alcohol
- Alcohol-Diclofenac interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- DICLOFENAC POTASSIUM®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "DICLOFENAC POTASSIUM- diclofenac potassium tablet, film coated".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Diclofenac |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Diclofenac |Label Name=Diclofenac11.png
}}
{{#subobject:
|Label Page=Diclofenac |Label Name=Diclofenac11.png
}}