Dextrose monohydrate injection (50%)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Adeel Jamil, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Dextrose monohydrate injection (50%) is a sterile intravenous solution that is FDA approved for the treatment of and indicated for use with automated compounding devices for preparing intravenous nutritional admixtures in the pharmacy. Common adverse reactions include hyperosmolar syndrome, febrile response, infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection, extravasation, and hypervolemia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- 50% and 70% Dextrose Injection, USP (concentrated dextrose in water) in Pharmacy Bulk Packages are indicated for use with automated compounding devices for preparing intravenous nutritional admixtures in the pharmacy.
Dosing Information
- Concentrated Dextrose in Water is administered by slow intravenous infusion (a) after admixture with amino acid solutions or (b) after dilution with other compatible IV fluids. Dosage should be adjusted to meet the requirements of each individual patient.
- 50% and 70% Dextrose Injection, USP in the 2000 mL flexible Pharmacy Bulk Package are designed for use with automated compounding devices for preparing intravenous nutritional admixtures. Dosages will be in accordance with the recommendation of the prescribing physician. 50% and 70% Dextrose Injection, USP are not intended for direct infusion. Admixtures should be made by, or under the direction of, a pharmacist using strict aseptic technique under a laminar flow hood. Compounded admixtures may be stored under refrigeration for up to 24 hours. Administration of admixtures should be completed within 24 hours after removal from refrigeration.
- The maximum rate at which dextrose can be infused without producing glycosuria is 0.5 g/kg of body weight/hr. About 95% of the dextrose is retained when infused at a rate of 0.8 g/kg/hr.
- Clinical evaluation and periodic laboratory determinations are necessary to monitor changes in fluid balance, electrolyte concentrations, and acid base balance during prolonged parenteral therapy or whenever the condition of the patient warrants such evaluation.
- As reported in the literature, the dosage and constant infusion rate of intravenous dextrose must be selected with caution in pediatric patients, particularly neonates and low birth weight infants, because of the increased risk of hyperglycemia/hypoglycemia.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Drug Interactions
- Additives may be incompatible with the fluid withdrawn from this container. Consult with pharmacist, if available. When compounding admixtures, use aseptic technique, mix thoroughly and do not store.
- Some opacity of the plastic due to moisture absorption during sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
Recommended Directions for Use of the Pharmacy Bulk Package Use Aseptic Technique
- During use, container must be stored, and all manipulations performed, in an appropriate laminar flow hood.
- Remove cover from outlet port at bottom of container.
- Insert piercing pin of transfer set and suspend unit in a laminar flow hood. Insertion of a piercing pin into the outlet port should be performed only once in a Pharmacy Bulk Package solution. Once the outlet site has been entered, the withdrawal of container contents should be completed promptly in one continuous operation. Should this not be possible, a maximum time of 4 hours from transfer set pin or implement insertion is permitted to complete fluid transfer operations; i.e., discard container no later than 4 hours after initial closure puncture.
- Sequentially dispense aliquots of 50% or 70% Dextrose Injection, USP into IV containers using appropriate transfer set. During fluid transfer operations, the Pharmacy Bulk Package should be maintained under the storage conditions recommended in the labeling.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Dextrose monohydrate injection (50%) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Dextrose monohydrate injection (50%) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Dextrose monohydrate injection (50%) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Dextrose monohydrate injection (50%) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Dextrose monohydrate injection (50%) in pediatric patients.
Contraindications
- A concentrated dextrose solution should not be used when intracranial or intraspinal hemorrhage is present nor in the presence of delirium tremens if the patient is already dehydrated.
- Dextrose injection without electrolytes should not be administered simultaneously with blood through the same infusion set because of the possibility that pseudoagglutination of red cells may occur.
Warnings
- Concentrated dextrose in water should be administered only after suitable dilution. Hypertonic dextrose solutions should be given slowly. Significant hyperglycemia and possible hyperosmolar syndrome may result from too rapid administration. The physician should be aware of the symptoms of hyperosmolar syndrome, such as mental confusion and loss of consciousness, especially in patients with chronic uremia and those with known carbohydrate intolerance.
- The intravenous administration of these solutions can cause fluid and/or solute overloading resulting in dilution of serum electrolyte concentrations, overhydration, congested states or pulmonary edema.
- WARNING: This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
- Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
FOR PERIPHERAL VEIN ADMINISTRATION
- Hypertonic dextrose solutions (above 5% concentration) should be given slowly, preferably through a small bore needle into a large vein, to minimize venous irritation.
FOR CENTRAL VENOUS ADMINISTRATION
- Concentrated dextrose should be administered via central vein after appropriate admixture or dilution when required.
PRECAUTIONS
- Electrolyte deficits, particularly in serum potassium and phosphate, may occur during prolonged use of concentrated dextrose solutions. Blood electrolyte monitoring is essential, and fluid and electrolyte imbalances should be corrected. Essential vitamins and minerals also should be provided as needed.
- To minimize hyperglycemia and consequent glycosuria it is desirable to monitor blood and urine glucose and if necessary, add insulin. When concentrated dextrose infusion is abruptly withdrawn, it is advisable to follow with the administration of 5% or 10% dextrose to avoid rebound hypoglycemia.
- Aseptic technique is essential with the use of sterile preparations for compounding nutritional admixtures. Discard container within 4 hours of entering closure.
- Solutions containing dextrose should be used with caution in patients with known subclinical or overt diabetes mellitus.
- Care should be exercised to insure that the needle (or catheter) is well within the lumen of the vein and that extravasation does not occur.
- Concentrated dextrose solutions should not be administered subcutaneously or intramuscularly.
- Do not administer unless solution is clear and container is undamaged.
Adverse Reactions
Clinical Trials Experience
- hyperosmolar syndrome, resulting from excessively rapid administration of concentrated dextrose may cause hypovolemia, dehydration, mental confusion and/or loss of consciousness.
- Reactions which may occur because of the solution or the technique of administration include febrile response, infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection, extravasation and hypervolemia.
- If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures and save the remainder of the fluid for examination if deemed necessary.
Postmarketing Experience
There is limited information regarding Dextrose monohydrate injection (50%) Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Dextrose monohydrate injection (50%) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Animal reproduction studies have not been conducted with dextrose. It is also not known whether dextrose can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Dextrose should be given to a pregnant woman only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Dextrose monohydrate injection (50%) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Dextrose monohydrate injection (50%) during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Dextrose monohydrate injection (50%) in women who are nursing.
Pediatric Use
- The safety and effectiveness in the pediatric population are based on the similarity of the clinical conditions of the pediatric and adult populations. In neonates or very small infants the volume of fluid may affect fluid and electrolyte balance. Caution should be exercised with low birth weight premature neonates, who are receiving dextrose concentrations of 10% or greater, as they are most susceptible to glucose intolerance and hyperglycemia. Frequent monitoring of serum glucose concentrations is required when dextrose is prescribed to pediatric patients, particularly neonates and low birth weight infants.
- In very low birth weight infants, excessive or rapid administration of dextrose injection may result in increased serum osmolality and possible intracerebral hemorrhage.
Geriatic Use
There is no FDA guidance on the use of Dextrose monohydrate injection (50%) in geriatric settings.
Gender
There is no FDA guidance on the use of Dextrose monohydrate injection (50%) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Dextrose monohydrate injection (50%) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Dextrose monohydrate injection (50%) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Dextrose monohydrate injection (50%) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Dextrose monohydrate injection (50%) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Dextrose monohydrate injection (50%) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
Monitoring
There is limited information regarding Dextrose monohydrate injection (50%) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Dextrose monohydrate injection (50%) and IV administrations.
Overdosage
- In the event of overhydration or solute overload during therapy, re-evaluate the patient and institute appropriate corrective measures.
Pharmacology
There is limited information regarding Dextrose monohydrate injection (50%) Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Dextrose monohydrate injection (50%) Mechanism of Action in the drug label.
Structure
- 50% and 70% Dextrose Injection, USP (concentrated dextrose in water) are sterile, nonpyrogenic, hypertonic solutions of Dextrose, USP in water for injection for intravenous administration after appropriate admixture or dilution.
- The Pharmacy Bulk Package is a sterile dosage form which contains multiple single doses for use only in a pharmacy bulk admixture program.
- The content and physical characteristics of the solutions are as follows:
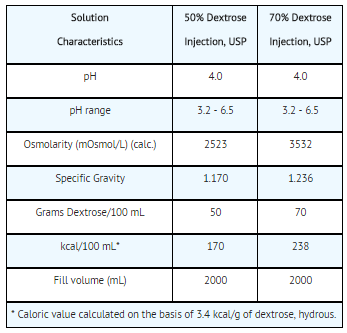
- The solutions contain no bacteriostat, antimicrobial agent or added buffer and are intended only for use as a single-dose injection following admixture or dilution.
- This Pharmacy Bulk Package is intended only for use in the preparation of sterile, intravenous nutrient admixtures using automated compounding devices. The flexible plastic container is fabricated from a specially formulated polyvinylchloride. Water can permeate from inside the container into the overwrap but not in amounts sufficient to affect the solution significantly. Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the plastic container materials. Exposure to temperatures above 25°C/77°F during transport and storage will lead to minor losses in moisture content. Higher temperatures lead to greater losses. It is unlikely that these minor losses will lead to clinically significant changes within the expiration period.
- Dextrose Injection, USP is a parenteral fluid and nutrient replenisher.
- Dextrose, USP is chemically designated D-glucose, monohydrate (C6H12O6 • H2O), a hexose sugar freely soluble in water. It has the following structural formula:

- Water for Injection, USP is chemically designated H2O.
Pharmacodynamics
- When administered intravenously, solutions containing carbohydrate in the form of dextrose restore blood glucose levels and provide calories. Carbohydrate in the form of dextrose may aid in minimizing liver glycogen depletion and exerts a protein sparing action. Dextrose injection undergoes oxidation to carbon dioxide and water.
- Water is an essential constituent of all body tissues and accounts for approximately 70% of total body weight. Average normal adult daily requirement ranges from two to three liters (1.0 to 1.5 liters each for insensible water loss by perspiration and urine production).
- Water balance is maintained by various regulatory mechanisms. Water distribution depends primarily on the concentration of electrolytes in the body compartments, and sodium (NA+) plays a major role in maintaining physiologic equilibrium.
Pharmacokinetics
There is limited information regarding Dextrose monohydrate injection (50%) Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Dextrose monohydrate injection (50%) Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Dextrose monohydrate injection (50%) Clinical Studies in the drug label.
How Supplied
50% and 70% Dextrose Injection, USP are supplied as follows:

Revised: June, 2010
Printed in USA EN-2527
Hospira, Inc., Lake Forest, IL 60045 USA
Storage
- Store at 20 to 25°C (68 to 77°F).
- Protect from freezing.
Images
Drug Images
{{#ask: Page Name::Dextrose monohydrate injection (50%) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Dextrose monohydrate injection (50%) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Dextrose monohydrate injection (50%) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Dextrose monohydrate injection (50%) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Dextrose monohydrate injection (50%) Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Dextrose monohydrate injection (50%) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.