Dextromoramide
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
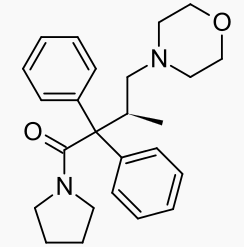
| Formula | C25H32N2O2 |
| Molar mass | 392.534 |
|
WikiDoc Resources for Dextromoramide |
|
Articles |
|---|
|
Most recent articles on Dextromoramide Most cited articles on Dextromoramide |
|
Media |
|
Powerpoint slides on Dextromoramide |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Dextromoramide at Clinical Trials.gov Trial results on Dextromoramide Clinical Trials on Dextromoramide at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Dextromoramide NICE Guidance on Dextromoramide
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Dextromoramide Discussion groups on Dextromoramide Patient Handouts on Dextromoramide Directions to Hospitals Treating Dextromoramide Risk calculators and risk factors for Dextromoramide
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Dextromoramide |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Dextromoramide (Palfium®, Palphium®, Jetrium®, Dimorlin®) is the right-handed isomer of the moramide molecule. The left handed molecule is called levomoramide, and a mixture of the two is called racemoramide. Its full chemical name is (+)-1-(3-Methyl-4-morpholino-2,2-diphenylbutyryl)pyrrolidine, and its molecular formula: C25H32N2O2, with an atomic weight of ~392.5. It is one of a group of molecules known as substituted α,α-Diphenyl-γ-Amino-Butyramides.
It is a powerful opioid analgesic, and as such is subject to drug prohibition regimes, both internationally through UN treaties, and by the criminal law of individual states.
It was discovered and patented in 1956 by Dr Paul Janssen at Janssen Pharmaceutica, who also discovered fentanyl, another important synthetic opioid, widely used to treat pain and in combination with other drugs as an anaesthetic. It has the proprietary name Palfium, though as of mid 2004 the drug was discontinued in the UK due to limited supplies of precursor chemicals.
As is the case with so many drug discoveries, dextromoramide was discovered by accident during research into almost identical compounds, namely α,α-Diphenyl-γ-Dialkyamino-Butyramides, which show no analgesic activity, but are extremely active physiologically as inhibitors of gastric secretions in man.
In many countries dextromoramide is unused, for example the USA, where it is considered a Schedule 1 drug, alongside cannabis and LSD, as having no medicinal uses. It is still rarely used in Australia, but prescription is avoided due to its abuse potential and so use of Palfium is now mainly limited to terminal care.
The typical dose is 5mg four hourly for cancer pain - the drug has a short half life comparable with pethidine. Dextromoramide is also used as a short-acting analgesic for minor surgical procedures. In this case it is administered sublingually and reaches a therapeutic concentration after around 12 minutes.
Dextromoramide was much favoured by drug abusers in Australia in the 1970s. A single case report and a review of the subject appears in the Australian Journal of Pharmacy 1980;61:641-644
References
{{reflist|2]]
- Den Otter G, Pain control with dextromoramide (pyrrolamidolum, R 875, palfium), Ned Tijdschr Geneeskd. 1958 Aug 23;102(34):1637-41.
- Lasagna L, De Kornfeld T, Safar P, A clinical trial of dextromoramide, (R 875, SKF D-5137), J Chronic Dis. 1958 Dec;8(6):689-93.
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Drug
- Opioids
- Morpholines
- Pyrrolidines