Desonide: Difference between revisions
No edit summary |
No edit summary |
||
| Line 54: | Line 54: | ||
|IVCompat=There is limited information about the IV Compatibility. | |IVCompat=There is limited information about the IV Compatibility. | ||
|overdose=Topically applied desonide cream and ointment can be absorbed in sufficient amounts to produce systemic effects. | |overdose=Topically applied desonide cream and ointment can be absorbed in sufficient amounts to produce systemic effects. | ||
|drugBox={{Drugbox2 | |||
| Verifiedfields = changed | |||
| Watchedfields = changed | |||
| verifiedrevid = 460778366 | |||
| IUPAC_name = (1''S'',2''S'',4''R'',8''S'',9''S'',11''S'',12''S'',13''R'')-11-hydroxy-8-(2-hydroxyacetyl)-6,6,9,13-tetramethyl-5,7-dioxapentacyclo[10.8.0.0<sup>2,9</sup>.0<sup>4,8</sup>.0<sup>13,18</sup>]icosa-14,17-dien-16-one | |||
| image = Desonide.png | |||
<!--Clinical data--> | |||
| tradename = Desowen | |||
| Drugs.com = {{drugs.com|monograph|desonide}} | |||
| MedlinePlus = a605025 | |||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | |||
| pregnancy_US = <!-- A / B / C / D / X --> C | |||
| pregnancy_category = | |||
| legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S8 --> | |||
| legal_UK = <!-- GSL / P / POM / CD --> | |||
| legal_US = <!-- OTC / Rx-only --> | |||
| legal_status = | |||
| routes_of_administration = topical | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = | |||
| protein_bound = | |||
| metabolism = | |||
| elimination_half-life = | |||
| excretion = | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 638-94-8 | |||
| ATC_prefix = D07 | |||
| ATC_suffix = AB08 | |||
| ATC_supplemental = {{ATC|S01|BA11}} | |||
| PubChem = 12536 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB01260 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 4470603 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = J280872D1O | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D03696 | |||
| ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ChEBI = 204734 | |||
| ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| ChEMBL = 1201109 | |||
<!--Chemical data--> | |||
| C=24 | H=32 | O=6 | |||
| molecular_weight = 416.507 g/mol | |||
| smiles = O=C\1\C=C/[C@]2(/C(=C/1)CC[C@H]3[C@H]4[C@](C[C@H](O)[C@H]23)([C@@]5(OC(O[C@@H]5C4)(C)C)C(=O)CO)C)C | |||
| InChI = 1/C24H32O6/c1-21(2)29-19-10-16-15-6-5-13-9-14(26)7-8-22(13,3)20(15)17(27)11-23(16,4)24(19,30-21)18(28)12-25/h7-9,15-17,19-20,25,27H,5-6,10-12H2,1-4H3/t15-,16-,17-,19+,20+,22-,23-,24+/m0/s1 | |||
| InChIKey = WBGKWQHBNHJJPZ-LECWWXJVBZ | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C24H32O6/c1-21(2)29-19-10-16-15-6-5-13-9-14(26)7-8-22(13,3)20(15)17(27)11-23(16,4)24(19,30-21)18(28)12-25/h7-9,15-17,19-20,25,27H,5-6,10-12H2,1-4H3/t15-,16-,17-,19+,20+,22-,23-,24+/m0/s1 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = WBGKWQHBNHJJPZ-LECWWXJVSA-N | |||
| synonyms = <small>(11β,16α)-11,21-Dihydroxy-16,17-[(1-methylethylidene)''bis''(oxy)]-pregna-1,4-diene-3,20-dione</small> | |||
}} | |||
|mechAction=Like other topical corticosteroids, desonide has anti-inflammatory, antipruritic and vasoconstrictive properties. The mechanism of the anti-inflammatory activity of the topical steroids, in general, is unclear. However corticosteroids are thought to act by the induction of phospholipase A2 inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A2. | |||
|structure=Desonide Cream, 0.05% and Ointment, 0.05% contain desonide (Pregna-1,4-diene-3,20-dione,11,21-dihydroxy-16,17-[(1-methylethylidene)bis(oxy)]-,(11β, 16α-)) a synthetic corticosteroid for topical dermatologic use. The corticosteroids constitute a class of primarily synthetic steroids used topically as anti-inflammatory and antipruritic agents. | |||
Chemically, desonide is C24H32O6. It has the following structural formula: | |||
[[File:Desonide.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
The molecular weight of desonide is 416.51. It is a white to off-white odorless powder which is soluble in methanol and practically insoluble in water. | |||
Each gram of Desonide Cream, 0.05% contains 0.5 mg of desonide in a compatible vehicle buffered to the pH range of normal skin. It contains aluminum acetate basic, cetearyl alcohol/SLS/SCS, glycerin, mineral oil, purified water, white petrolatum and white wax. It is preserved with methylparaben. | |||
Each gram of Desonide Ointment, 0.05% contains 0.5 mg of desonide in an ointment base consisting of mineral oil and white petrolatum. It is a smooth, uniform petrolatum-type ointment. | |||
|PK=The extent of percutaneous absorption of topical corticosteroids is determined by many factors, including the vehicle and the integrity of the epidermal barrier. Occlusive dressings with hydrocortisone for up to 24 hours have not been demonstrated to increase penetration; however, occlusion of hydrocortisone for 96 hours markedly enhances penetration. Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin may increase percutaneous absorption. | |||
Studies performed with desonide cream and ointment indicate that they are in the low range of potency as compared with other topical corticosteroids. | |||
|nonClinToxic=Long-term animal studies have not been performed to evaluate the carcinogenic, mutagenic, or fertility impairment potential of Desonide Cream, 0.05% and Ointment, 0.05%. | |||
|clinicalStudies=FDA Package Insert for Desonide contains no information regarding Clinical studies. | |||
|howSupplied=Desonide Cream 0.05% is supplied in 15 g (NDC 51672-1280-1) and 60 g (NDC 51672-1280-3) tubes. | |||
Desonide Ointment 0.05% is supplied in 15 g (NDC 51672-1281-1) and 60 g (NDC 51672-1281-3) tubes. | |||
|storage=Store at 20°- 25°C (68°- 77°F) [see USP Controlled Room Temperature]. Protect from freezing. | |||
|fdaPatientInfo=Patients using topical corticosteroids should receive the following information and instructions: | |||
1. This medication is to be used as directed by the physician. It is for external use only. Avoid contact with the eyes. | |||
2. This medication should not be used for any disorder other than that for which it was prescribed. | |||
3. The treated skin area should not be bandaged, or otherwise covered or wrapped, so as to be occlusive unless directed by the physician. | |||
4. Patients should report to their physician any signs of local adverse reactions. | |||
|alcohol=Alcohol-Desonide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Desonide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
|brandNames=* Desowen | |||
* LoKara | |||
* Tridesilon | |||
* Verdeso | |||
* Desonate | |||
|lookAlike=There is limited information about the look alike drug names. | |||
}} | |||
{{LabelImage | |||
|fileName=Desonide_label_01.jpg | |||
}} | |||
{{LabelImage | |||
|fileName=Desonide_label_02.jpg | |||
}} | |||
{{LabelImage | |||
|fileName=Desonide_panel_01.png | |||
}} | |||
{{LabelImage | |||
|fileName=Desonide_panel_02.png | |||
}} | }} | ||
Revision as of 19:04, 15 September 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Desonide is a Corticosteroid that is FDA approved for the treatment of inflammatory and pruritic manifestations of corticosteroid responsive dermatoses. Common adverse reactions include Contact dermatitis, Dry skin, Pruritus, Stinging of skin, Burning sensation, Irritation symptom.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Dermatoses
- Dosing information
- Desonide cream or ointment should be applied to the affected areas as a thin film two or four times daily depending on the severity of the condition.
- As with other corticosteroids, therapy should be discontinued when control is achieved. If no improvement is seen within two weeks, reassessment of diagnosis may be necessary.
- Desonide cream and ointment should not be used with occlusive dressings.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Desonide in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Desonide in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness in pediatric patients have not been established
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Desonide in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Desonide in pediatric patients.
Contraindications
Desonide cream and ointment are contraindicated in those patients with a history of hypersensitivity to any of the components of the preparations.
Warnings
General
Systemic absorption of topical corticosteroids can produce reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for glucocorticosteroid insufficiency after withdrawal of treatment. Manifestations of Cushing's syndrome, hyperglycemia, and glucosuria can also be produced in some patients by systemic absorption of topical corticosteroids while on treatment. Patients applying a topical steroid to a large surface area or to areas under occlusion should be evaluated periodically for evidence of HPA axis suppression. This may be done by using the ACTH stimulation, A.M. plasma cortisol, and urinary free cortisol tests. Patients receiving superpotent corticosteroids should not be treated for more than two weeks at a time and only small areas should be treated at any one time due to the increased risk of HPA suppressions. One of ten patients treated for one week under occlusion (30% of body surface) with Desonide Cream, 0.05% developed HPA axis suppression as determined by metapyrone testing. No specific studies relevant to potential HPA suppression have been conducted with Desonide Ointment, 0.05%. If HPA axis suppression is noted, an attempt should be made to withdraw the drug, to reduce the frequency of application, or to substitute a less potent corticosteroid. Recovery of HPA axis function is generally prompt upon discontinuation of topical corticosteroids. Infrequently, signs and symptoms of glucocorticosteroid insufficiency may occur requiring supplemental systemic corticosteroids. For information on systemic supplementation, see prescribing information for those products. Pediatric patients may be more susceptible to systemic toxicity from equivalent doses due to their larger skin surface to body mass ratios (See PRECAUTIONS - Pediatric Use). If irritation develops, desonide cream or ointment should be discontinued and appropriate therapy instituted. Allergic contact dermatitis with corticosteroids is usually diagnosed by observing a failure to heal rather than noting a clinical exacerbation as with most topical products not containing corticosteroids. Such an observation should be corroborated with appropriate diagnostic patch testing. If concomitant skin infections are present or develop, an appropriate antifungal or antibacterial agent should be used. If a favorable response does not occur promptly, use of desonide cream or ointment should be discontinued until the infection has been adequately controlled. Desonide Cream, 0.05% and Ointment, 0.05% should not be used in the presence of infection at the treatment site, hypersensitivity to corticosteroids, or pre-existing skin atrophy. Desonide Cream, 0.05% and Ointment, 0.05% should not be used in the eyes. FOR EXTERNAL USE ONLY.
Laboratory Tests The following tests may be helpful in evaluating patients for HPA axis suppression:
- ACTH stimulation test
- A.M. plasma cortisol test
- Urinary free cortisol test
Adverse Reactions
Clinical Trials Experience
In controlled clinical trials, the total incidence of adverse reactions associated with the use of Desonide Cream, 0.05% was approximately 1% and Desonide Ointment, 0.05% was approximately 6%. The adverse reactions for Desonide Cream, 0.05% were pruritus, pain, folliculitis, rash, peripheral edema, pustular rash, sweating, erythema, irritation, and burning. Laboratory abnormalities were found in 3% of the patients. These were hyperglycemia (2%) and liver function abnormality (1%). The adverse reactions for Desonide Ointment, 0.05% were erythema, induration, pruritus, irritation, oiliness, and peripheral edema. The following additional local adverse reactions have been reported infrequently with other topical corticosteroids, and they may occur more frequently with the use of occlusive dressings and higher potency corticosteroids. These reactions are listed in approximate decreasing order of occurrence: dryness, folliculitis, acneiform eruptions, perioral dermatitis, allergic contact dermatitis, secondary infection, skin atrophy, striae, miliaria, burning and hypopigmentation.
Postmarketing Experience
FDA Package Insert for Desonide contains no information regarding Postmarketing experience .
Drug Interactions
FDA Package Insert for Desonide contains no information regarding Drug Interaction.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C
Corticosteroids have been shown to be teratogenic in laboratory animals when administered systemically at relatively low dosage levels. Some corticosteroids have been shown to be teratogenic after dermal application in laboratory animals. Animal reproductive studies have not been conducted with desonide cream or ointment. It is also not known whether desonide cream or ointment can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. There are no adequate and well-controlled studies in pregnant women. Desonide cream or ointment should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Desonide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Desonide during labor and delivery.
Nursing Mothers
Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in human milk. Because many drugs are excreted in human milk, caution should be exercised when desonide cream or ointment is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established. Because of a higher ratio of skin surface area to body mass, pediatric patients are at a greater risk than adults of HPA axis suppression and Cushing's syndrome when they are treated with topical corticosteroids. They are therefore also at greater risk of adrenal insufficiency during or after withdrawal of treatment. Adverse effects including striae have been reported with inappropriate use of topical corticosteroids in infants and children. HPA axis suppression, Cushing's syndrome, linear growth retardation, delayed weight gain and intracranial hypertension have been reported in children receiving topical corticosteroids. Manifestations of adrenal suppression in pediatric patients include low plasma cortisol levels, and absence of response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema.
Geriatic Use
There is no FDA guidance on the use of Desonide in geriatric settings.
Gender
There is no FDA guidance on the use of Desonide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Desonide with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Desonide in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Desonide in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Desonide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Desonide in patients who are immunocompromised.
Administration and Monitoring
Administration
Applied to the affected area.
Monitoring
FDA Package Insert for Desonide contains no information regarding Drug Monitoring.
IV Compatibility
There is limited information about the IV Compatibility.
Overdosage
Topically applied desonide cream and ointment can be absorbed in sufficient amounts to produce systemic effects.
Pharmacology
Mechanism of Action
Like other topical corticosteroids, desonide has anti-inflammatory, antipruritic and vasoconstrictive properties. The mechanism of the anti-inflammatory activity of the topical steroids, in general, is unclear. However corticosteroids are thought to act by the induction of phospholipase A2 inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A2.
Structure
Desonide Cream, 0.05% and Ointment, 0.05% contain desonide (Pregna-1,4-diene-3,20-dione,11,21-dihydroxy-16,17-[(1-methylethylidene)bis(oxy)]-,(11β, 16α-)) a synthetic corticosteroid for topical dermatologic use. The corticosteroids constitute a class of primarily synthetic steroids used topically as anti-inflammatory and antipruritic agents. Chemically, desonide is C24H32O6. It has the following structural formula:
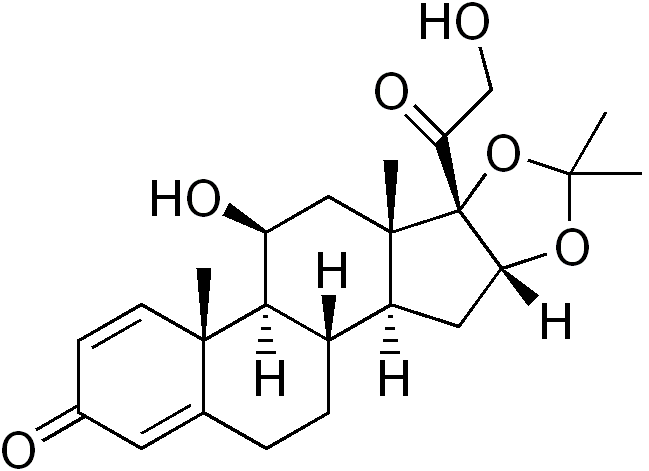
The molecular weight of desonide is 416.51. It is a white to off-white odorless powder which is soluble in methanol and practically insoluble in water. Each gram of Desonide Cream, 0.05% contains 0.5 mg of desonide in a compatible vehicle buffered to the pH range of normal skin. It contains aluminum acetate basic, cetearyl alcohol/SLS/SCS, glycerin, mineral oil, purified water, white petrolatum and white wax. It is preserved with methylparaben. Each gram of Desonide Ointment, 0.05% contains 0.5 mg of desonide in an ointment base consisting of mineral oil and white petrolatum. It is a smooth, uniform petrolatum-type ointment.
Pharmacodynamics
There is limited information regarding Desonide Pharmacodynamics in the drug label.
Pharmacokinetics
The extent of percutaneous absorption of topical corticosteroids is determined by many factors, including the vehicle and the integrity of the epidermal barrier. Occlusive dressings with hydrocortisone for up to 24 hours have not been demonstrated to increase penetration; however, occlusion of hydrocortisone for 96 hours markedly enhances penetration. Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin may increase percutaneous absorption. Studies performed with desonide cream and ointment indicate that they are in the low range of potency as compared with other topical corticosteroids.
Nonclinical Toxicology
Long-term animal studies have not been performed to evaluate the carcinogenic, mutagenic, or fertility impairment potential of Desonide Cream, 0.05% and Ointment, 0.05%.
Clinical Studies
FDA Package Insert for Desonide contains no information regarding Clinical studies.
How Supplied
Desonide Cream 0.05% is supplied in 15 g (NDC 51672-1280-1) and 60 g (NDC 51672-1280-3) tubes. Desonide Ointment 0.05% is supplied in 15 g (NDC 51672-1281-1) and 60 g (NDC 51672-1281-3) tubes.
Storage
Store at 20°- 25°C (68°- 77°F) [see USP Controlled Room Temperature]. Protect from freezing.
Images
Drug Images
{{#ask: Page Name::Desonide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Desonide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Patients using topical corticosteroids should receive the following information and instructions:
1. This medication is to be used as directed by the physician. It is for external use only. Avoid contact with the eyes. 2. This medication should not be used for any disorder other than that for which it was prescribed. 3. The treated skin area should not be bandaged, or otherwise covered or wrapped, so as to be occlusive unless directed by the physician. 4. Patients should report to their physician any signs of local adverse reactions.
Precautions with Alcohol
Alcohol-Desonide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Desowen
- LoKara
- Tridesilon
- Verdeso
- Desonate
Look-Alike Drug Names
There is limited information about the look alike drug names.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Desonide |Label Name=Desonide_label_01.jpg
}}
{{#subobject:
|Label Page=Desonide |Label Name=Desonide_label_02.jpg
}}
{{#subobject:
|Label Page=Desonide |Label Name=Desonide_panel_01.png
}}
{{#subobject:
|Label Page=Desonide |Label Name=Desonide_panel_02.png
}}